A Case of Acute Macular Neuroretinopathy and its Association with COVID-19
Purpose: This case report showcases Acute Macular Neuroretinopathy (AMN), a rare condition of vascular occlusive origin. It also highlights a possible association between AMN and COVID-19 infections and vaccinations.
Material and Methods: A 23-year-old female with complaints of unusual visual phenomena was examined several times at an optometry school clinic. Fundus images were obtained using ultra-wide field imaging. Optical coherence tomography (OCT) and OCT Angiography (OCT-A) were used to track macular structural and vascular changes. Visual fields were obtained to assess for central defects. A literature search was conducted for possible associations between COVID-19 and AMN.
Results: The patient was initially diagnosed with ocular migraines to explain visual phenomena of “shimmering orbs”, exacerbated by a history of concussions. However, after performing vision therapy exercises with no relief of symptoms, further investigation supported the diagnosis of AMN associated with previous oral contraceptive use and viral infection.
There have been over 100 case reports of this condition until 2016. Over the past two years, 25 case reports have proposed associations between AMN diagnosis and COVID-19 infection or vaccinations.
Conclusions: Although rare, AMN should be on the list of differential diagnoses if patients may be experiencing central visual phenomenon; multimodal imaging is key to this diagnosis. More research must be conducted to determine the pathophysiology of AMN, along with its relationship with COVID-19 infection or vaccination.
Introduction
A 23-year-old female with history of concussions and subsequent occipital headaches and eyestrain presented with a complaint of central shimmering “orbs” slightly temporal to the visual axis in each eye, greater in the left than the right eye. The patient started experiencing this symptom after oral contraceptive use and a viral infection in college. Although initially diagnosed as ocular migraines, further testing revealed a retinal etiology. Acute Macular Neuroretinopathy (AMN) is a rare microvascular retinal disorder that typically affects white females, predominantly in the third decade of life. The disorder typically manifests as outer retinal dysfunction with associated scotomas and decreased or blurred vision. Additionally, it can be associated with flu-like symptoms/fever or oral contraceptive use.1 Although formal diagnostic criteria have not been established, multimodal imaging, especially optical coherence tomography (OCT) and OCT Angiography (OCT-A), is key to aiding in the diagnosis of AMN, as it demonstrates structural abnormalities that may not be visible to ophthalmoscopy.
Material and Methods
A 23-year-old female patient was examined several times at a university eye clinic. Fundus images were obtained using a Daytona Optos ultra-wide field laser retinal imager (Optos PLC, Dunfermline, Scotland, United Kingdom). OCT and OCT-A scans were used to track macular structural and vascular changes with a Zeiss Cirrus 5000 with AngioPlex (Carl Zeiss Meditec AG, Jena, Germany). Visual fields were used to assess for central defects with the Haag-Streit Octopus 900 Pro (Haag Streit AG, Koeniz, Switzerland). Fluorescein angiography was used to assess retinal perfusion with a Zeiss FF450plus with VISUPAC (Carl Zeiss Meditec AG, Jena, Germany). A literature search was conducted on Google Scholar and PubMed with keywords “COVID-19” or “SARS CoV-2” and “AMN” or “Acute Macular Neuroretinopathy.”
Results
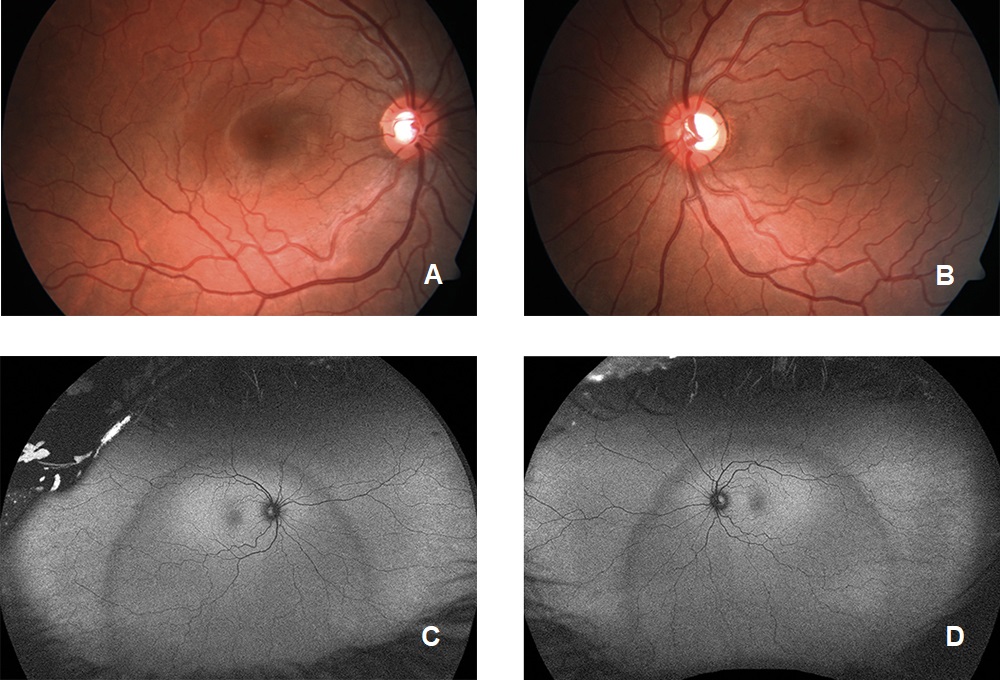
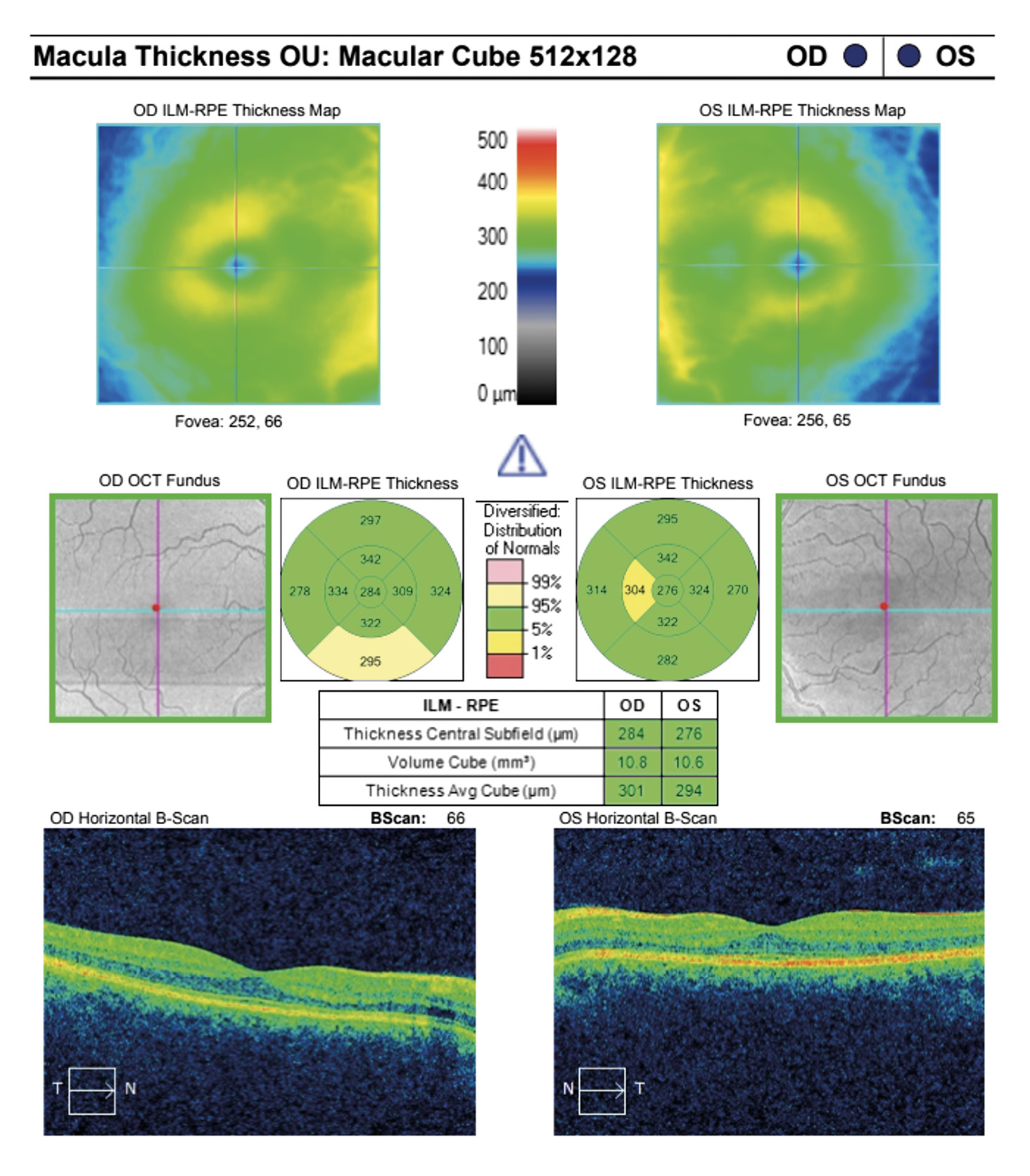
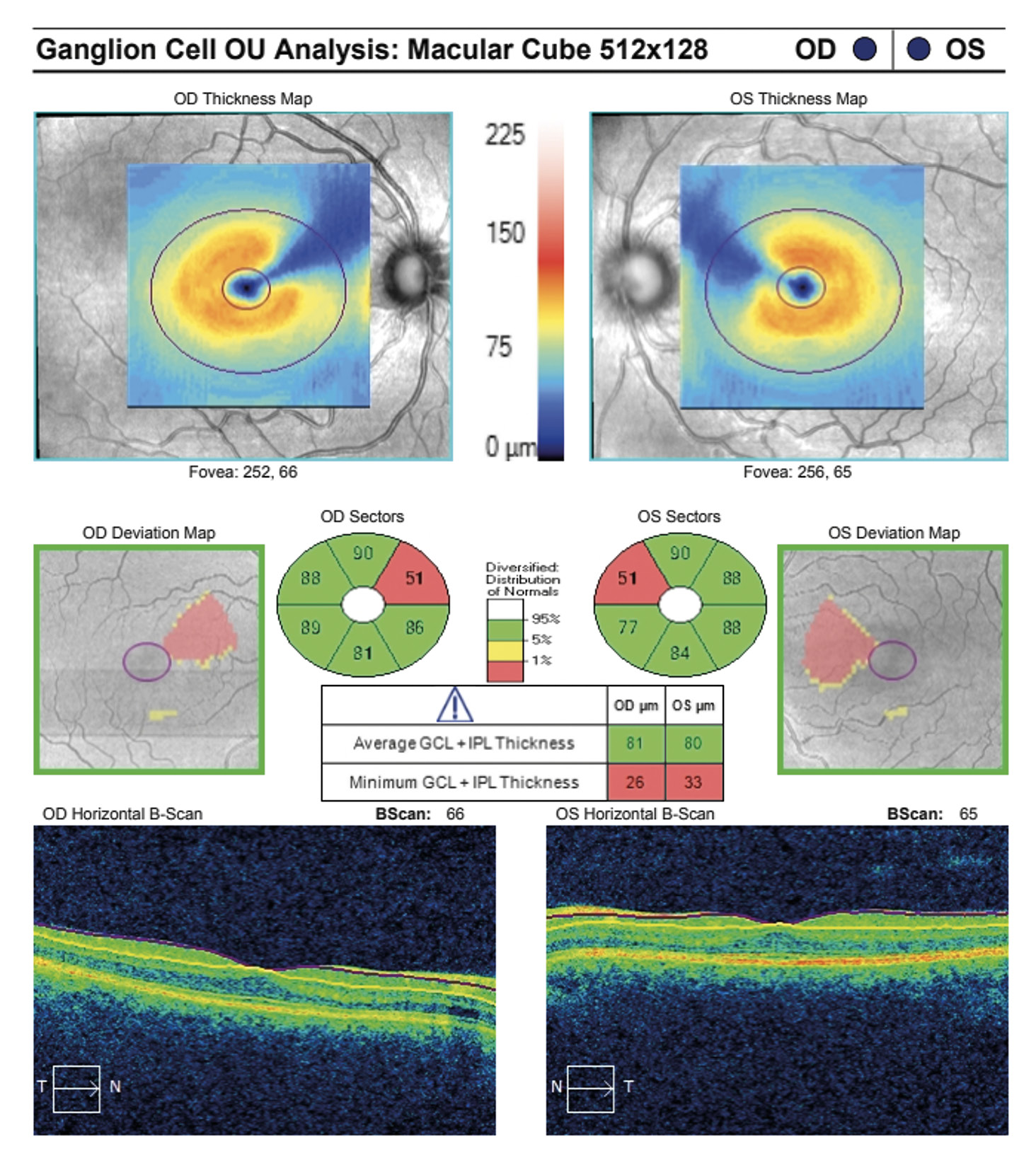
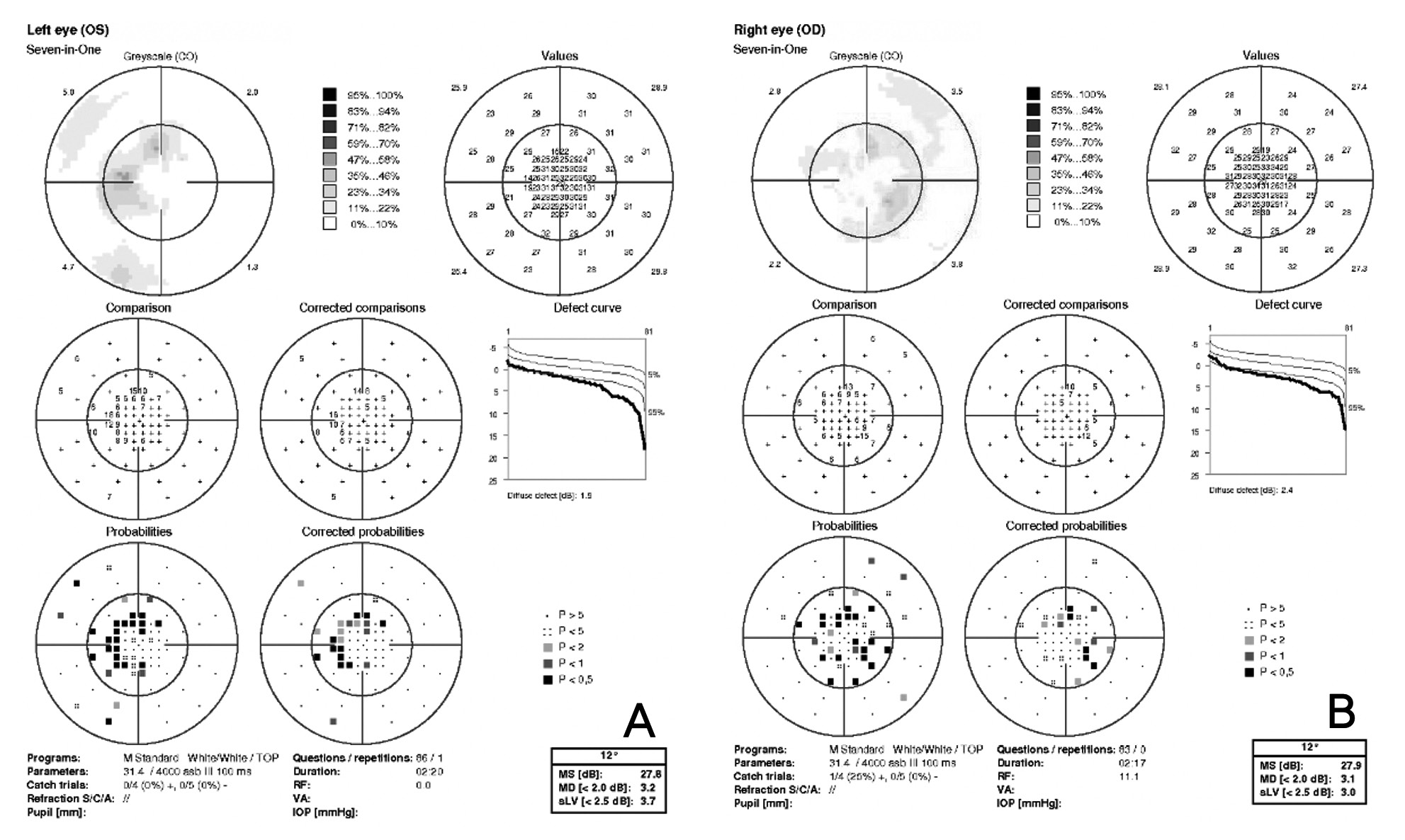
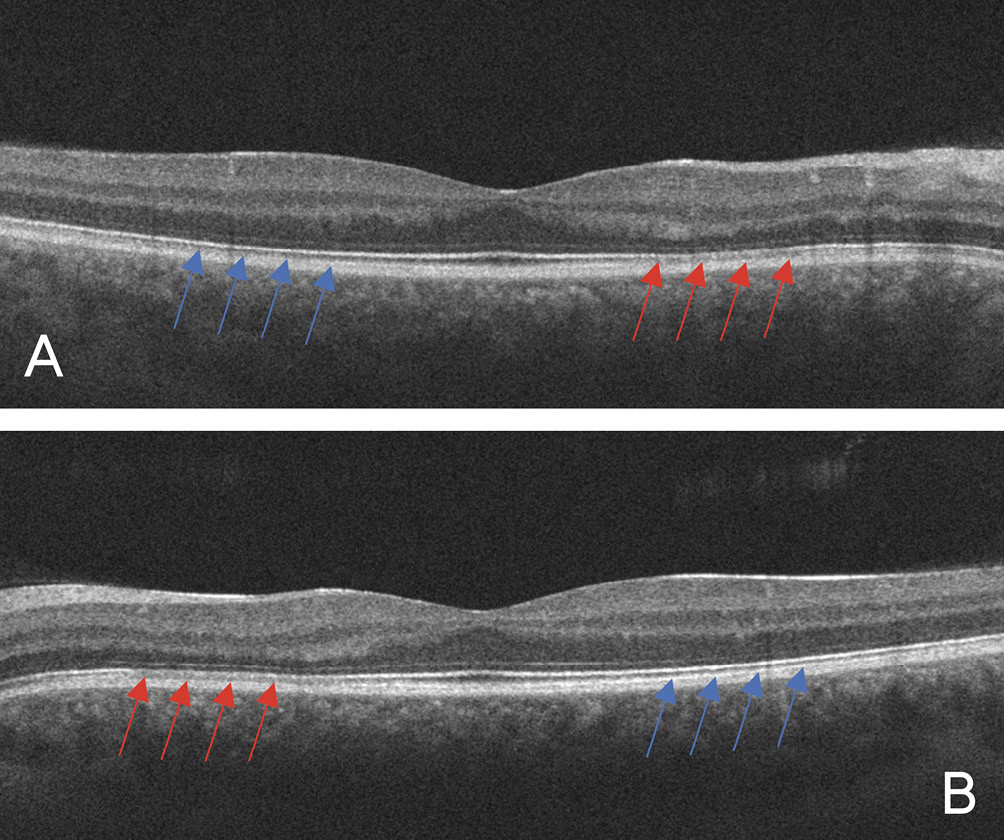
Visual acuity without correction was 20/20 in the right and left eye. Dilated fundus examination revealed larger than average optic nerves with corresponding enlarged cup to disc ratios and symmetrical vascular tortuosity; otherwise, the retina was unremarkable with a positive foveal reflex in each eye (Figure 1). OCT demonstrated no abnormal macular thickness (Figure 2) or retinal nerve fiber layer (RNFL) thinning in either eye, but ganglion cell analysis revealed symmetrical superior nasal ganglion cell layer/inner plexiform layer (GCL+IPL) thinning in both eyes and slight GCL+IPL thinning inferiorly (Figure 3). Visual fields showed corresponding dense defects temporal to fixation (Figure 4). OCT 5-line raster scans showed bands of hyper-reflectivity in the outer plexiform layer (OPL) nasal to the foveal center with underlying disruption in the ellipsoid zone (EZ)/ photoreceptor integrity line (PIL) (Figure 5), while en-face OCT-A scans showed darkened retinal lesions signifying lack of vascular flow in the inner segment (IS)/outer segment (OS) and choriocapillaris (CC) segments (Figure 6). All abnormal findings were greater in the left eye which remained consistent with the patient’s symptomatology. Magnetic resonance imaging of brain and orbits with and without contrast were ordered to rule out space occupying lesions, and fluorescein angiography (FA) was performed to assess for perfusion; both of which were unremarkable. Based upon the examination and imaging results, the patient was diagnosed with Acute Macular Neuroretinopathy (AMN).
Discussion
AMN is a rare microvascular retinal disorder that typically affects white females, predominantly in the third decade of life. Around half of the known cases are bilateral, with a majority reporting central visual phenomenon and/or decreased vision. Although formal diagnostic criteria have not been established, typical features in the acute phase may include reddish-brown wedge-shaped retinal lesions whose apices point towards the foveal center while in others the lesions are not clinically appreciable.3 Some cases have shown symptom resolution, while others have had lasting symptomatology for months afterwards. Typical associations with AMN include non-specific fever and malaise and oral contraceptive use. Less common associations include exposure to epinephrine or ephedrine, previous trauma, systemic shock, IV contrast exposure, pre-eclampsia, post-partum hypotension, and caffeine consumption.1
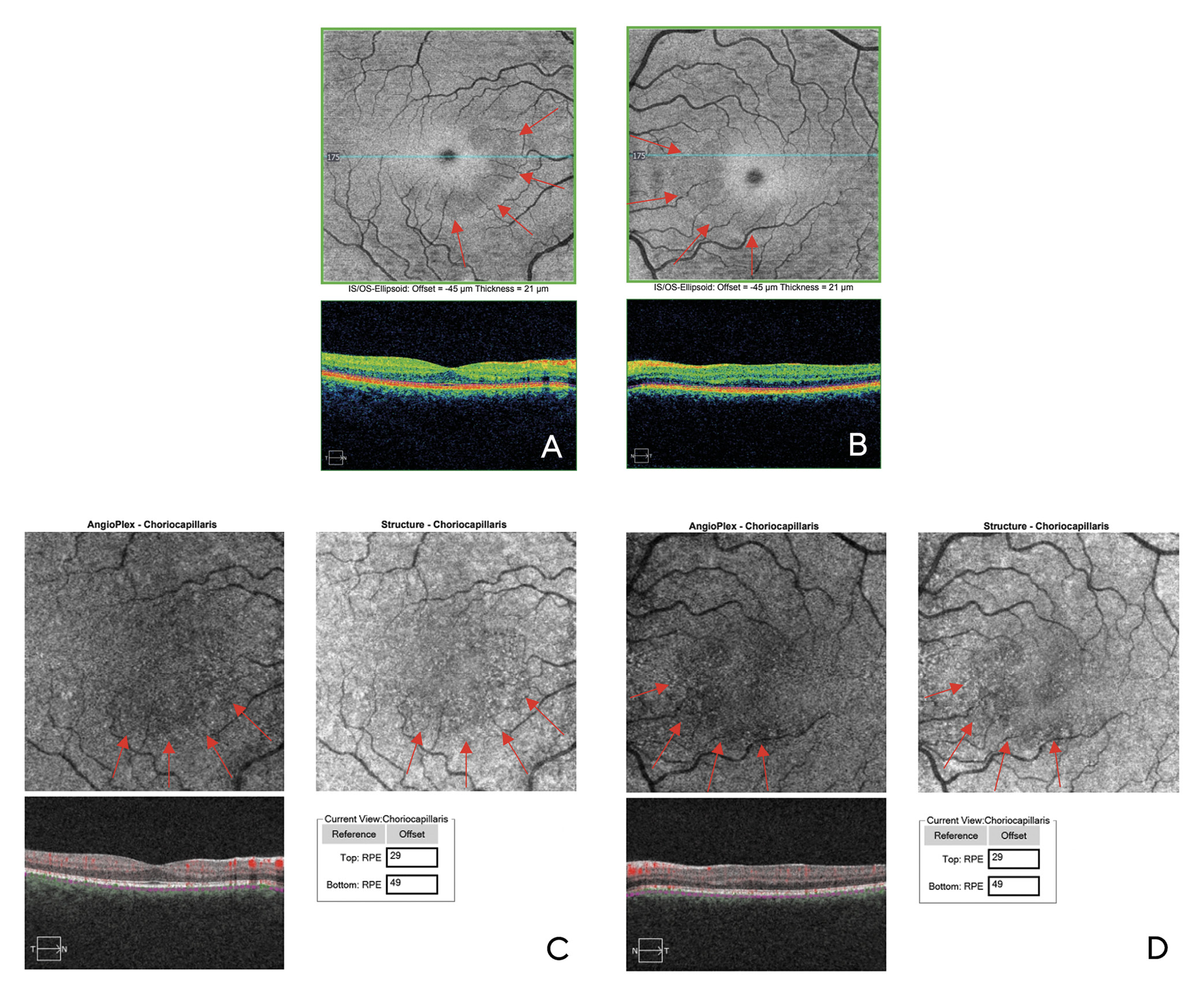
Multimodal imaging aids in the diagnosis of AMN, with OCT and OCT-A being the key tests, the latter of which is used to find areas of low vascular flow. OCT findings in the acute phase may show hyper-reflective bands beginning in the OPL and the outer nuclear layer (ONL) with underlying disruption of the EZ/PIL and retinal pigment epithelium (RPE); these changes can attenuate over time.2 En-face OCT-A of the corresponding areas are hypo-reflective which indicate lack of vascular flow; they typically are most prominent in the IS/OS and/or CC layers. Fundus autofluorescence (FAF) imaging can also delineate AMN lesions, though with lower reliability than OCT. Visual fields will help confirm central scotomas. FA studies are unremarkable in AMN but can be helpful to rule out other pathology.1
Although the exact etiology of AMN has not been elucidated, researchers have found a vascular occlusive component to this disorder.1 OCT-A imaging studies have uncovered the multi-laminated vascular structures that supply the retina which are consistent with previous primate models. In a 2018 study by Nesper and Fawzi, an enhanced OCT-A segmentation algorithm was used to divide the parafovea into three plexuses, each of which has their own anastomosis:3
1) The superficial capillary plexus (SCP), located in the RNFL and the ganglion cell layer (GCL)
2) The middle capillary plexus (MCP), located at the boundary of the inner plexiform layer (IPL) and the inner nuclear layer (INL)
3) The deep capillary plexus (DCP), located at the boundary of the INL and OPL
Although defects have been demonstrated in both the DCP and underlying CC, there is debate as to which is the initial area of damage in AMN. The OPL has been shown to be the first layer affected in AMN, suggesting the initial area of insult is the DCP. Additionally, AMN does not show appreciable change on FA, suggesting that the CC is uninvolved.1 Although the CC has been shown to be the area of insult in some OCT-A studies, some researchers argue that there is a shadowing artifact from the overlying DCP which may erroneously give the appearance of CC involvement.4 By contrast, a study by Casalino et.al. showed that although intralesional CC and DCP vessel densities were significantly decreased in subjects with AMN vs. controls, there was an overall significant decrease in overall vessel density in the CC as compared to the DCP in AMN patients.5 Further research must be conducted to determine the area of initial insult in AMN.
AMN and Covid-19
In the recent setting of the COVID-19 pandemic, there have been 25 case reports associating COVID-19 vaccination6-17 or infection18-27 with the diagnosis of AMN; the exact relationship between them has yet to be established. These case reports propose an association between COVID-19 vaccination and AMN, especially the Vaxzevria (AstraZeneca) formulation (Table 1). AstraZeneca vaccines have been associated with increases in thrombotic related adverse effects as compared with Moderna and Pfizer formulations, especially for those between 18-64 years old. Increases in serum coagulability factors were noted 10 days after AstraZeneca vaccination, which could contribute to the microvascular occlusion seen in AMN.28
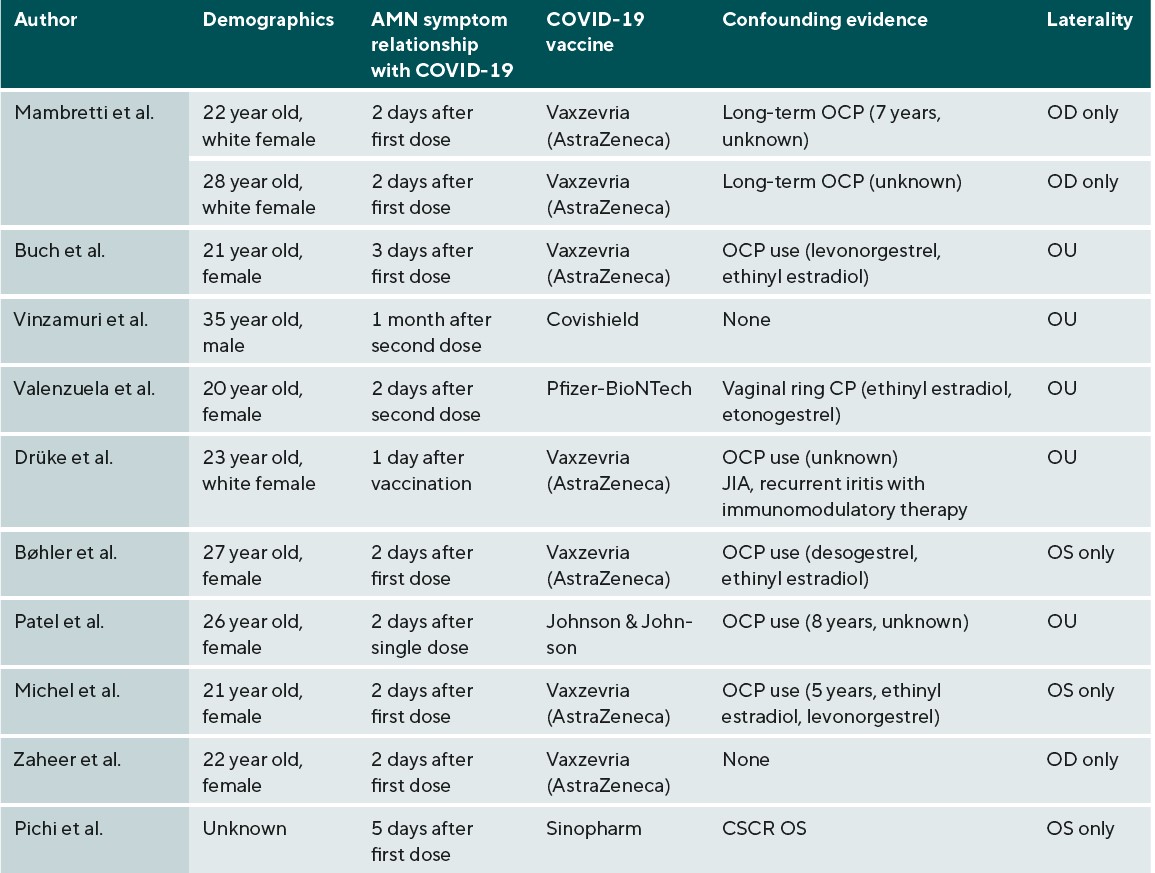
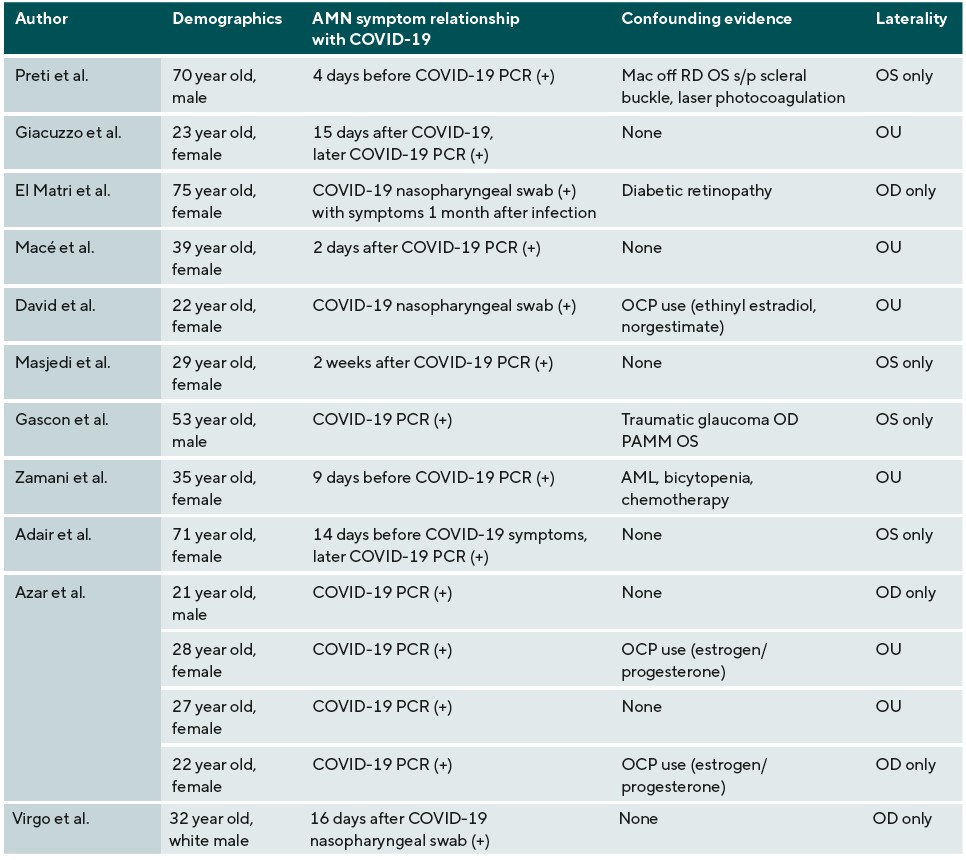
Case reports have also associated AMN with patients who have contracted COVID-19 (Table 2). Studies have shown an inflammatory association between COVID-19 and the renin-angiotensin system, in which SARS CoV-2 separates angiotensin converting enzyme 2 (ACE-2) from its membranal attachment, disseminating the virus. ACE-2 is responsible for making angiotensin 1-7, a protein that counteracts the vasoconstricting and pro-inflammatory properties of angiotensin II.29 ACE-2 receptors are expressed in the GCL, INL, IPL, and OS and have been shown to be down-regulated with progression of diabetic retinopathy.30 Oxygen measurements through animal models have shown that the IPL and the OPL are the most oxygen-demanding, which likely correlates with synaptic activity. The DCP, which supplies blood flow to these areas, is more structurally susceptible to micro-vascular events as compared to the MCP or SCP.3 A confounding factor with AMN and COVID-19 vaccination is oral contraceptive (OCP) use; this confounding factor is much less prevalent in the COVID-19 infection case reports. Combined hormonal contraceptives (CHC), which have estrogen and progesterone components, have been shown to decrease anti-coagulant factors and increase pro-coagulation factors and risk of arterial complications. A study by Rabiolo et.al. found that young females with long-term CHC use had increased retinal arterial constriction under dynamic vessel analysis as compared to controls.31 Estrogen and progesterone receptors are in the choroid and retina, and studies have also shown OCP-related alterations in the macula and choroidal thickness.32 Although already a risk factor for AMN, this may have an additive effect on the pro-inflammatory effects of COVID-19. Moreover, confounding conditions in both groups which include central serous chorioretinopathy,14 traumatic glaucoma,25 diabetic retinopathy,13 juvenile idiopathic arthritis (JIA), immunomodulatory therapy,6 acute myeloid leukemia (AML), and chemotherapy23 all either have inflammatory components or increase the opportunity for inflammation to occur and in theory can also increase the risk of AMN.
There are several limitations to this case report. Since the exact pathophysiology of AMN is unknown, it is impossible to draw any correlations between AMN and COVID-19 infection or vaccination. The number of case reports proposing associations between COVID-19 vaccination or infection and AMN are small considering the vast population that have been vaccinated or infected with COVID-19, however this correlates with the low prevalence of AMN. ACE-2 receptors are down-regulated in diabetic retinopathy, however, there has not been evidence as to why AMN has not been associated with diabetic retinopathy. In addition, there is a lack of evidence as to why OCP usage was a more prevalent confounding factor in the COVID-19 vaccination case reports over those of COVID-19 infection. This discrepancy may be due to the increase in SARS-CoV-2 in vascular circulation with COVID-19 infection that could increase the risk of microvascular complications; more research must be conducted to determine its significance. Three case reports proposing an association between COVID-19 infection and AMN diagnosis had a positive COVID-19 test after being diagnosed with AMN, which may decrease the reliability of an association between these two conditions.
Conclusion
Although rare, AMN should be on the list of differential diagnoses when patients experience central visual phenomena. Multimodal imaging, particularly OCT and OCT-A, is key to this diagnosis. More research must be conducted to determine the exact etiology of the condition and its relationship with COVID-19 infection or vaccination.
Conflict of interest
The author declares that there is no conflict of interests regarding the methods and devices mentioned in the article.
COE Multiple Choice Questionnaire
The publication "A Case of Acute Macular Neuroretinopathy and its Association with COVID-19" has been approved as a COE continuing education article by the German Quality Association for Optometric Services (GOL). The deadline to answer the questions is 01. January 2024. Only one answer per question is correct. Successful completion requires answering four of the six questions.
You can take the continuing education exam while logged in.
Users who are not yet logged in can register for ocl-online free of charge here.
