Concomitant Keratoconus and Fuchs’ Endothelial Dystrophy- A Case Report
Purpose: The present report describes a rare case of concomitant Keratoconus (Kc) and Fuchs’ Endothelial Dystrophy (FED) and how this patient was managed to achieve a 20/20 OU visual outcome post-multiple ocular surgeries.
Methods and Results: A Caucasian male in his early 40’s with Kc and FED OU entered our clinic wearing low Dk/t hybrid lenses. Subsequently and after penetrating keratoplasty (PKP) in both eyes and Descemet’s stripping automated endothelial keratoplasty (DSAEK) in the right eye, the patient was prescribed scleral lenses (SLs) OU, providing good comfort, physiology and vision (20/25 OD; 20/20 OS). Endothelial cell count at the most recent visit was 1248 c/mm2 OD and 2354 c/mm2 OS. The right eye was prescribed the Custom Stable Elite SL from Valley Contax with overall diameter 15.8 mm, Optimum Extreme material (Dk/t 125) and HydraPEG coating, and the left eye the ZenLens SL from Alden Optical (Bausch & Lomb) with overall diameter 16.0 mm, Boston XO2 material (Dk/t 141) and HydraPEG coating.
Conclusions: This patient’s corneas were challenged by endothelial disease provoking an excessive reduction in cell density leading to the need for bilateral transplant surgeries. Due to the concurrently present Kc a PKP procedure was selected for both eyes. A subsequent episode of graft rejection (OD) was treated therapeutically but necessitated a DSAEK procedure. Management with high Dk/t SLs permitted this patient to enjoy good, functional vision at distance and near, excellent lens wearing comfort and, under the circumstances, sustainable corneal health.
Introduction
Fuchs’ Endothelial Dystrophy (FED) and Keratoconus (Kc) occurring concomitantly in the same cornea is rare and presents the contact lens practitioner with a cornea challenged by disease at both its anterior and posterior sides. The incidence of Kc is often stated to be 1 in 20001. However, a recent study utilizing 4.4 million patient records from a mandatory health insurance database in The Netherlands arrived at a prevalence of Kc to be 1 in 377 (95% confidence interval: 260-270).2 In contrast, when Kc occurs together with FED, the incidence is approximately 1 in 100,000.3 Although Kc was first described more than 150 years ago, we still do not know exactly where or how this corneal disease starts.4 However, at least initially, it is known to be an anterior corneal disease involving epithelium, its basement membrane, anterior limiting lamina (ALL) and stroma5 (Fig. 1.0). Only in its more advanced stages does Kc involve the posterior aspects of the cornea, as is seen in corneal hydrops, where breaches in the posterior limiting lamina (PLL) and endothelium allow a free influx of aqueous into the corneal stroma. The gradual but ultimately complete loss of ALL explains the frequent incidence of epithelial staining in Kc patients because the ALL provides the foundation on which the epithelium is anchored. The thickening and incomplete nature of epithelial basement membrane in the Kc cornea further compromises epithelial adhesion to ALL exacerbating epithelial fragility.
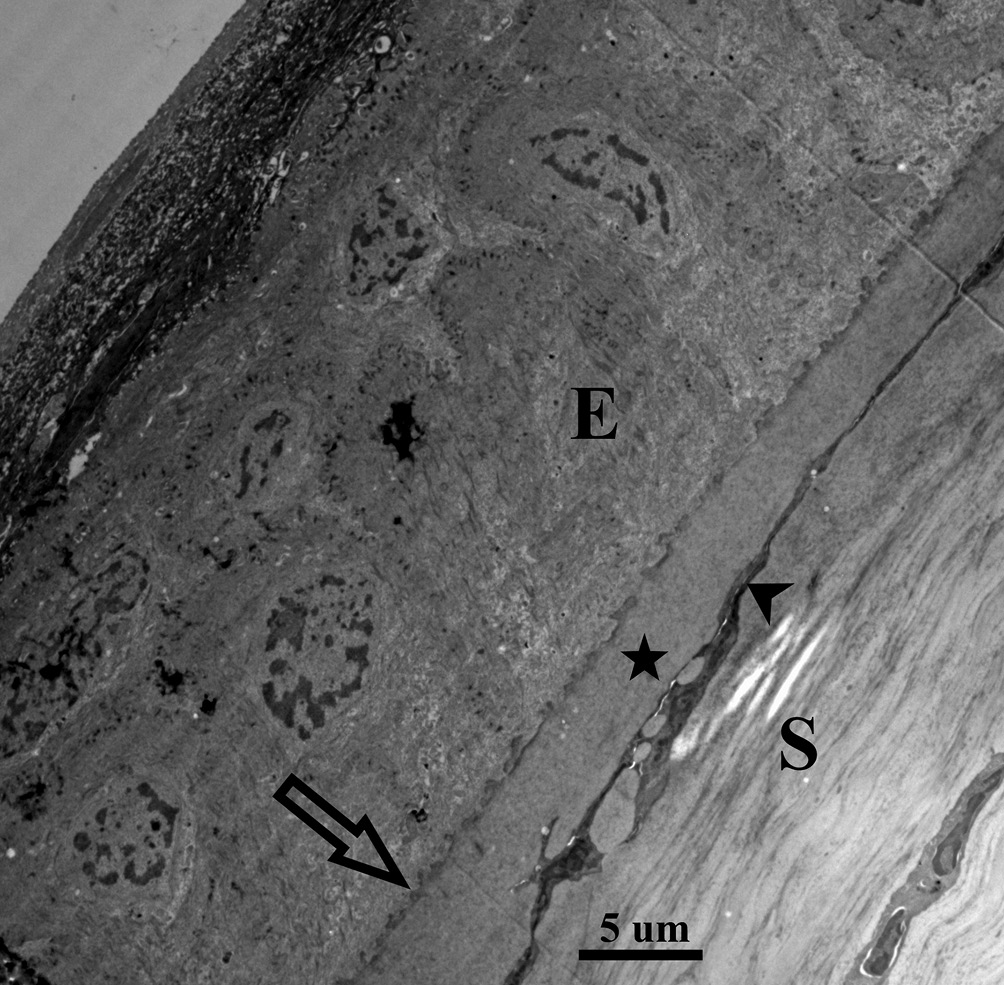
In contrast, FED is entirely explained by an endothelial dysfunction involving endothelial over-production of basement membrane. The endothelium is already the proficient basement membrane builder in the body adding approximately 1 um thickness to the PLL per decade, but in the case of FED this production becomes excessive.6 Furthermore, this abnormal basement membrane synthesis goes into uncontrolled hyperactivity at intervals creating mushroom-like basement membrane piles, known clinically as gutta (plural guttae), which leads to endothelial cell loss (Figure 2.1, 2.2). In other words, in FED the endothelial cells are engaged in a self-destructing activity. This enormous buildup of abnormal type IV collagen into large heaps, guttae, pushes the delicate endothelial cells around until they simply drop out and the patient ends up with a low endothelial cell count.7,8 With a cell count of less than 500 – 1000 cells per mm2, the cornea does not have enough cells to cover its posterior surface or enough cells to accomplish the endothelial function of pumping fluid back into the anterior chamber, or both.9 With an insufficient number of endothelial cells present, edema and corneal decompensation will ensue. Each one of these conditions causes a decrease in visual acuity but in different ways. Kc leads to irregular astigmatism and resultant higher order aberrations often with accompanying apical scarring that decreases corneal transparency while FED causes visual changes due to the compromise of corneal deturgescence, resulting in corneal edema and consequent clouding of the cornea.10 The present case report describes the complex surgical and contact lens management when these corneal diseases occur in the same patient.
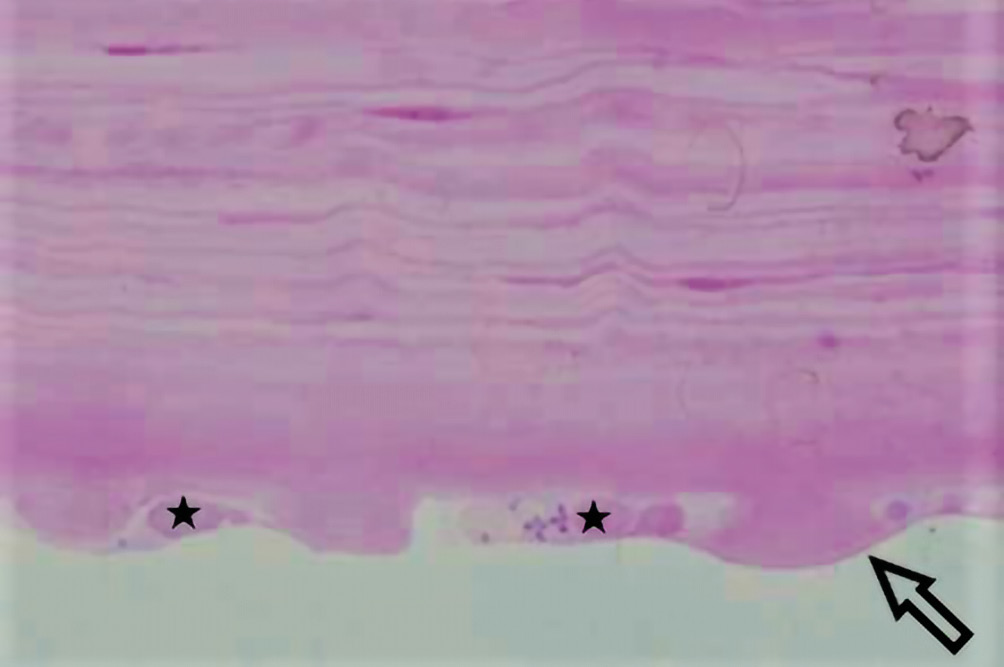

Methods and Results
The patient, a Caucasian male, now in his early 40’s was first diagnosed with Kc in his second decade of life and FED in his third decade. He was referred to our clinic in his early 30’s and wearing low Dk/t hybrid lenses (SynergEyes, Carlsbad, California, USA- specific modal not known) prescribed by a previous doctor. He had a complaint of discomfort and visual blur with his current lenses. Best corrected visual acuity was 20/40 OD, 20/30 OS at distance. At this visit, biomicroscopy revealed grade 3+ guttae and mild corneal haze OU, and Fleischer’s ring OS. Corneal topography measured K values for the right eye as 51.2 D/46.7 D and a thinnest pachymetry reading of 459 um. The left eye K values were 51.3 D/46.3 D and the thinnest pachymetry reading was 459 um. The apex of the ectasia for both eyes was slightly displaced inferior and temporal. The habitual lenses moved insufficiently with blinks. The patient was prescribed scleral lenses (SLs) OU at this visit to provide better comfort, increased oxygen transmittance, and an improved physical fit. Lens details are listed below:
- OD: ZenLens (Bausch & Lomb, Lancaster, New York, USA)/ Prolate/ 7.8 mm/0.00-0.75x055 D/17.0 mm/ Boston XO (100Dk/t material)
- OS: ZenLens (Bausch & Lomb, Lancaster, New York, USA)/ Prolate/ 7.8 mm/0.00 D/17.0 mm/ Boston XO (100Dk/t material)
One month later at the dispense appointment, the patient returned to the clinic with the chief complaint of glare and halos. Examination revealed epithelial bullae in the inferior-nasal cornea OD and grade 3+ guttae OU. Visual acuity with the new scleral lenses was 20/40 OD, 20/30 OS at distance. The patient was advised to start using Muro-128 5% ointment OU at bedtime and Muro-128 5% solution OU every six hours for two weeks. He was instructed to delay scleral lens wear for the right eye. Approximately four months later, the patient had a penetrating keratoplasty (PKP) OD. During that month, scleral lens wear was continued only with the left eye. At the next visit four months later, biomicroscopy revealed a clear PKP graft OD and grade 3+ guttae OS. Five months further on, the patient had a successful PKP OS. Scleral lens wear was halted after surgery until both eyes were cleared for safe contact lens wear. Uncorrected visual acuity was 20/30 OD and 20/80 OS.
Two years following the bilateral PKP surgeries, the patient exhibited graft rejection in the right eye and was treated by the corneal surgeon who had performed his transplants. He was prescribed ophthalmic steroids (Durezol – 3 times/day for 1st wk, followed by 2 times/day until resolution) for the right eye. The rejection symptoms resolved in one month, resulting in a clear and quiet graft. Distance best corrected visual acuity with manifest refraction was 20/40 OD and 20/30 OS. Endothelial cell count was 1546 c/mm2 OD and 2435 c/mm2 OS (NIDEK Specular Microscope CEM-530). Corneal topography measured K values for the right eye as 44.1 D/44.9 D and a thinnest pachymetry reading of 586 um. The left eye K values were 39.6 D/48.9 D and the thinnest pachymetry reading was 564 um.
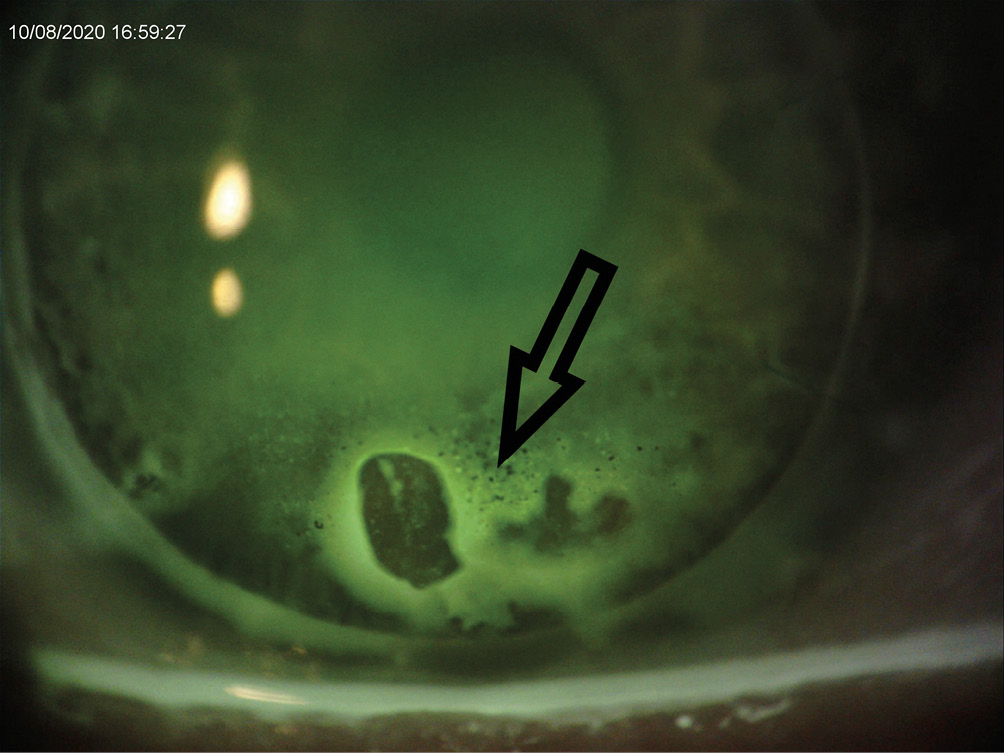
Twelve months after the rejection symptoms resolved in the right eye, the patient was cleared to be re-fitted with scleral lenses in both eyes. At that time, both PKP grafts were clear with no signs of rejection. Specular microscopy was performed and the endothelial cell count was 1101 c/mm2 OD and 2284 c/mm2 OS. Scleral lenses resulted in best corrected visual acuity of 20/20 OD and 20/25 OS. Almost four months after resuming scleral lens wear, the patient presented for another scleral lens follow-up and complained of discomfort in his right eye. Biomicroscopy revealed microcystic epithelial edema on the lower 1.5 mm of the PKP graft with keratic precipitates OD. The endothelial cell count in that eye had dropped further to 879 c/mm2. Visual acuity was stable at 20/20 OD and 20/25 OS. At that time, the patient was referred back to the corneal surgeon who advised discontinuation of scleral lens wear in the right eye and monitoring of the condition. Later that year, four years post-PKPs, the patient returned to the clinic with a large sub-epithelial bulla and microcystic epithelial edema OD, and a clear and quiet graft OS (Figure 3.0). Endothelial cell count was now 500 c/mm2 OD and 2372 c/mm2 OS. Pachymetry values revealed a corneal thickness of 646 um OD and 566 um OS. Distance visual acuity with habitual scleral lenses was 20/40 OD and 20/20 OS .The patient was referred back to the corneal specialist for a graft evaluation. Following a few months of monitoring, the patient had a DSAEK performed on his right eye.
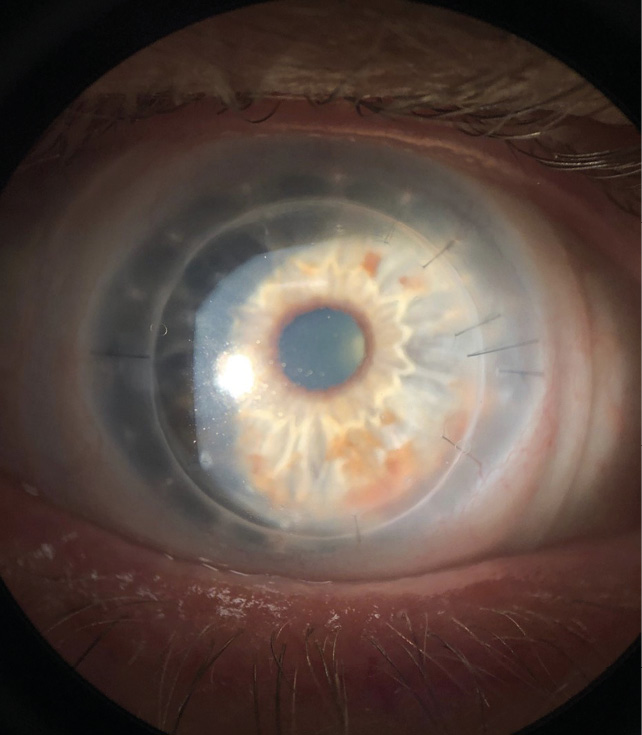
When the patient’s eyes were stable, he was re-fitted in scleral lenses for both eyes. Biomicroscopy revealed a clear DSAEK OD, and a clear PKP OS. The endothelial cell count was 1248 c/mm2 OD and 2354 c/mm2 OS. Best corrected visual acuity with scleral lenses was 20/25 OD and 20/20 OS. The prescribed scleral lenses resulted in good vision, and comfort. The lenses provided a central clearance of about 150 um, with adequate clearance in the mid-periphery and limbal areas in order to safely protect the graft-host interface for each eye. (Figure 4.1, 4.2). The patient’s right eye had permanent sutures and it was important to ensure that the scleral lens did not touch or rub against them. A scleral lens is our preferred option to provide clearance of the vulnerable surgical interface. A monovision fit was achieved (with +2.00 D over OD) which resulted in 20/20 vision OU at distance and near. The final lens details are listed below:
- OD: Valley Contax, Custom Stable Elite (Springfield, Oregon, USA)8.23 mm/+4.50 D/15.8 mm/ CT 0.360 mm/Optimum Extreme (Dk/t 125) w/HydraPEG
- OS: Alden Optical, Zenlens (Bausch & Lomb, Lancaster, New York), USA)/ZenLens Oblate/8.50 mm/+4.50 D/16.0 mm/ CT 0.490 mm/Boston XO2 (Dk/t 141) w/HydraPEG
Discussion
The self-destructive endothelial behavior in FED leads to a decline in endothelial cell density. In a cornea challenged with both FED and Kc, a PKP is the best surgical option. In the present case, an episode of graft rejection compromised the transplanted endothelium. The graft rejection pathophysiological process led to uncontrolled edema and accelerated endothelial cell loss that required a DSAEK. FED without concomitant disease is today best managed surgically with DSAEK, since this procedure only involves the removal of the endothelium with its basement membrane – PLL. This procedure is superior to the PKP surgery, which was the standard approach in the past. In the case of FED, PKP removes 500 um of healthy tissue to replace the pathological 3-5 um thick endothelium with its abnormal basement membrane (8-12 um). In contrast, DSAEK leaves the healthy anterior cornea intact, which translates into a quicker healing time and a maintenance of global strength, which is crucial in the case of blunt trauma. DSAEK also causes minimal change in refractive error. It is well recognized that severed corneal lamellae will never heal and, therefore, a PKP leaves an eye permanently vulnerable to trauma.11 Patients with a PKP must be counseled on this fact and be advised to always wear protective glasses or goggles when a contact is possible, as in many sports.
In this case, where the corneas harbored two pathologies affecting different corneal extremes, the surgical management dictated a PKP procedure, which later, on the right eye, had a DSAEK procedure. It is proposed here that the need for an additional endothelial transplant was due to the accelerated endothelial cell loss associated with surgery and inflammation rather than from a re-emergence of the disease, as can happen in transplant surgeries for Kc.12,13 The most recent cell count on the left eye indicated an excellent cell density of 2354 c/mm2, while the right eye had 1248 c/mm2, which is good but monitoring is advisable. Both eyes were wearing SLs and the higher endothelial cell density in OS than OD ruled out the SL wear as a cause of the cell loss in the right eye. Patients with FED and Kc, even when they occur concomitantly, which is rare, can successfully be managed with contact lenses. Depending on the severity of Kc, there are several contact lens options available to help patients achieve better vision when spectacle correction is inadequate. Specialty and toric soft contact lenses, especially with a high modulus, may be adequate for cases of mild Kc. Hybrid lenses, piggyback, or corneal RGP lenses may be successful for moderate Kc. For cases of moderate to severe Kc with a high amount of irregular astigmatism and aberrations, SLs are typically the best lens option to consider in order to achieve maximum vision potential. SLs are well accepted by Kc patients who achieve good comfort and vision with these devices and it has been reported that the wear of SLs delays the need for keratoplasty in this population.14,15 Post-keratoplasty, the SL has been reported to provide excellent visual rehabilitation with few complications.16
The contact lens management of a patient with multiple ocular transplant surgeries may require two different lens designs, as was the case with our patient. Given the compromised endothelial history of the current case, it was decided that a high Dk/t material was warranted. The 16.0 mm and 15.8 mm overall diameters of the lenses was indicated as this patient had relatively narrow palpebral fissures and easy and safe insertion and removal was a concern. A Hydra-PEG coating was added to the lenses to enhance wettability and patient comfort with sustained wear throughout the day. This patient has worn these SL designs with excellent vision and comfort for two years. Due to his visual demands, the lenses were prescribed to afford monovision allowing the patient to see 20/20 at distance and near with both eyes open. Monovision was selected because of his profession as a lawyer and being a presbyope. As of his most recent visit to the clinic, his transplant status remains stable. There have not been any further signs of rejection and his endothelial cell counts have remained stable. In summary, despite the presence of two separate corneal pathologies and multiple ocular transplant surgeries, this patient achieved comfortable all day wear with his SL prescription with a 20/20 visual outcome at distance and near.
Conflict of interest
The authors have no conflict of interest with regard to the methods and devices mentioned in the article.
COE Multiple Choice Questionnaire
The publication "Concomitant Keratoconus and Fuchs’ Endothelial Dystrophy- A Case Report" has been approved as a COE continuing education article by the German Quality Association for Optometric Services (GOL). The deadline to answer the questions is 01 November 2023. Only one answer per question is correct. Successful completion requires answering four of the six questions.
You can take the continuing education exam while logged in.
Users who are not yet logged in can register for ocl-online free of charge here.
