The impact of diabetes mellitus and hyperglycaemia on the refractive status of the eye
Purpose: Diabetes mellitus is a condition of considerable concern globally, which can affect the visual system in various ways, including changes to visual function, the integrity of the ocular surface and the retinal microcirculation. The aim of this article is to provide an overview on the perspectives around the relationship between diabetes and refractive status.
Material and Methods: Narrative literature review.
Results: The relationship between diabetes, hyperglycaemia and refractive error has been of interest to clinicians and researchers for more than a century. This review shows that research studies investigating the relationship have varied considerably in their design, methodology, their outcome measures used as well as their reported results. While some uncertainty remains, there is evidence that short-term, drastic changes in blood glucose levels affect the refractive status of human eyes leading to fluctuating and blurred vision.
Conclusion: Patients starting glycaemic treatment or undergoing adaptation to a new treatment regime may present with considerable refractive changes and visual complaints. Before considering the prescription of spectacle lenses, clinicians should ideally monitor patients in whom glycaemic control has been initiated or is being adjusted until a stabilisation of blood glucose levels has been confirmed.
Introduction
Obtaining information on diabetes mellitus (DM) from patients is a standard element of history taking prior to refractive assessment and clinical examination of ocular health. Asking questions about DM allows practitioners to assess the risk of diabetic retinopathy and to put any refractive complaints, which may be due to poorly controlled hyperglycaemia, into context. The latter is especially important if a patient has reported a recent temporary fluctuation in vision, which constitutes a common complaint in patients with DM and hyperglycaemia.
Epidemiology and types of diabetes mellitus
DM is a chronic systemic condition and a primary cause of morbidity and mortality globally. The estimated prevalence in adults worldwide in 2019 was 422 million (8.5%) and around 1.5 million deaths were attributed to the condition.1,2 There are three different types of DM including type 1, type 2 and gestational diabetes. DM type 1 is rarer than DM type 2 and associated with deficient insulin production, leading to a need for daily injections of insulin. The causes of DM type1 are still uncertain and there is no known prevention available. DM type 2 is considerably more common than DM type 1 and characterised by what is thought an ineffective use of insulin by the body. It has been reported that approximately one quarter of adults in the United States have been diagnosed with DM type 2.3 This type has been shown to be associated with obesity and physical inactivity as well as genetic factors and processes related to ageing.3 Although DM type 2 used to be a condition that was primarily diagnosed in adults, the World Health Organization (WHO) reports that it is now more frequently detected in children. Gestational DM refers to elevated blood sugar levels (hyperglycaemia) above normal values, but below levels of DM, during pregnancy. This type is considered by the WHO as a risk factor for the development of DM type 2 later in life.1
Hyperglycaemia
All types of DM carry health risks that are associated with hyperglycaemia, which describes an imbalance between glucose production by the body (liver), glucose uptake through nutrition and glucose uptake by target tissues such as muscle. The imbalance leads to greater than normal glucose levels, which are used to diagnose DM and to monitor treatment efficacy.3 To monitor changes, fasting plasma glucose as well as haemoglobin A1c (HbA1c) levels can be measured.4
Effect of diabetes and hyperglycaemia on ocular structures
The consequences of DM range from mild systemic and ocular findings to severe and life-threatening complications. These complications include microvascular changes, stroke, blindness, coronary heart disease, kidney disease and amputations.2 Research studies have investigated the impact of DM on ocular structures and functions. Several effects on the ocular surface have been reported, including reduced tear film stability and secretion and reduced corneal sensitivity.5 Central corneal thickness has been shown to be increased in DM6,7 and poor glycaemic control can lead to decreased corneal endothelial cell density.7 A recent major review confirmed that people living with DM have altered corneal endothelial morphology such as increased pleomorphism, polymegathism and decreased endothelial cell density. These structural changes appear to be associated with functional changes including reduced endothelial pump and barrier functions, leading to greater corneal thickness and hypoxic stress.8
Dry eye has been reported to be common in DM type 29,10 and to be related to a reduction in quality of life.10 Studies reviewing the associations between DM and peripheral changes have suggested that corneal nerve changes due to DM present an opportunity for the early detection of peripheral neuropathy and early treatment.11
Hyperglycaemia has been shown to cause diabetic keratopathy, but also to be a cause of retinal and choroidal cell death.12 Recently, lower corneal optical density has been reported in people with DM in comparison to non-diabetic individuals.13 In contrast to the negative impact DM can have on the ocular surface, the condition is not thought to be a significant risk factor for glaucomatous optic neuropathy.14
However, DM does not only affect the ocular surface and the anterior segment of the ocular system. Diabetic retinopathy (DR) is a major and potentially sight threatening microvascular complication of DM affecting the posterior segment. DR represents the leading cause of preventable blindness in people of working age,15 affecting about a third of people with DM.16 Hyperglycaemia is commonly the underlying factor for the development of DR, even though there is a long list of associated risk factors including hypertension, dyslipidaemia, DM duration and ethnic origin. 15 Early detection of DR is paramount to minimise visual impairment. To facilitate early detection, diabetic retinal screening programmes are in operation in many countries and have been shown to be successful and effective in identifying people developing DR and associated complications such as visual impairment. In Scotland, a national Diabetic Retinal Screening programme was rolled out in 2006. People with DM aged 12 years and older are invited to attend either community or hospital-based retinal screening clinics (note: these are not ophthalmology clinics). Following a brief patient history and assessment of habitual and best-corrected visual acuity, fundus photographs are obtained and evaluated to identify clinical signs of DR.17 Patients with non-proliferative forms of DR are monitored at regular intervals at screening clinics, e.g. at 6- or 12-months intervals. People with more severe presentations and potentially proliferative DR or signs of maculopathy are referred for detailed examination at the ophthalmology hospital department. Overall, about 4% of patients require ophthalmology referral, (once the screening programme has been fully established). The most common cause for ophthalmology referral is macular oedema.17
With both, anterior and posterior ocular structures affected by DM, it seems reasonable and logical to assume that structural changes may have a noticeable impact on the refractive status of the visual system. These functional changes are primarily due to underlying pathophysiology that is linked to biochemical changes in response to hyperglycaemia.
Aims
This invited review was devised to provide a succinct overview of the current knowledge and key aspects of DM and its impact on the refractive system in adult humans. A particular focus was placed on acute changes in refractive status.
Methods
Literature search strategy, inclusion and exclusion criteria
A literature search was carried out on 25 April 2020, using a set of keywords including ‘diabetes’, ‘refraction’, ‘refractive error’, ‘myopia’, ‘hyperopia’ and keyword combinations to scrutinise the electronic database of the National Library of Medicine (MEDLINE) through the EBSCO host access at a university library. MEDLINE is a major database and currently contains more than 27 million references to journal articles in life sciences from more than 5,200 journals, ranging from the year 1966 to the present day. Articles published in English language and covering the topic of this review were included. A manual search was conducted for relevant systematic reviews on the topic, including the Cochrane Database of Systematic Reviews. The website of the World Health Organization was searched for relevant reports and general patient information on diabetes mellitus.
Results
The focus of this review has been on the effect of DM on refraction, which has been considered for at least a century. Table 1 provides a summary of the included studies and highlights the various outcome measures that have been reported in studies which investigated the relationship between refractive error and DM. The studies included in this review are presented in ascending chronological order by year of publication.
An early example is a paper by Duke-Elder, who presented a series of three cases that were examined at St. George’s Hospital in London in the 1920s. In this case series, it is suggested that a reduction in blood glucose could lead to hyperopic refractive error and an increase in glucose levels can lead to a more myopic refractive status.18 The first of these cases was a patient who was admitted to hospital with severe symptoms of DM and the refractive changes (hyperopic shift) were observed suddenly within a day and followed the start of insulin therapy, which led to temporary hypoglycaemia. The acute hyperopic shift was found to be reversible, but also quite variable during the period in which the insulin dosage (and blood glucose) was adjusted.
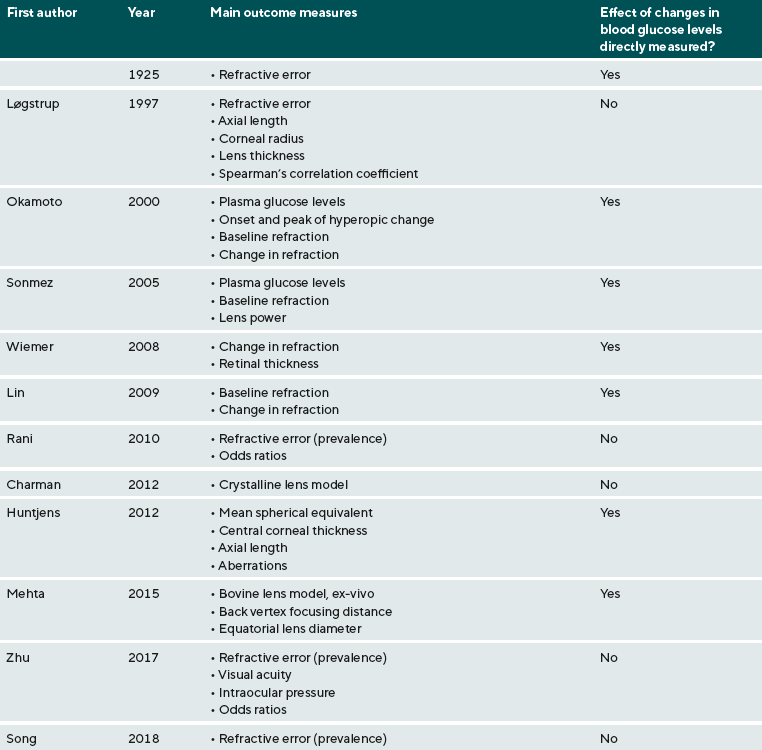
The second case also described a patient who was admitted to hospital in a severe, DM-related health state. Following initiation of insulin treatment, a considerable hyperopic shift with considerable astigmatism was observed. Similar to the first case, this acute change in refraction normalised once blood glucose levels had been stabilised and normalised. The third case was a long-term diabetic patient who was also suffering from what appeared to have been severe underlying health problems. In this patient, a drastic myopic shift was observed alongside an increase in blood glucose. Even though no meaningful statistical analysis can be undertaken based on these three cases, the paper nevertheless presents a useful insight into early observations of refractive shifts in patients with acute changes in blood glucose levels.
More recently, a Danish study was conducted to investigate the impact of DM on refraction in twins.19 Data were obtained from the Danish Twin Register and a total of 43 twin pairs were examined. A key outcome of the study was the observation that studies of relations between refraction and duration of DM showed diverging results. In the monozygotic (MZ) group, a tendency to reduced axial length and corresponding hyperopia with increasing duration of DM was found. However, in the dizygotic (DZ) group of same sex twins the opposite tendency was found. Increasing lens thickness and decreasing anterior chamber depth with increasing DM duration have been confirmed in this study. The authors conclude that insulin-dependent DM may influence refractive status on different levels.19
Okamoto and colleagues observed refractive changes during intensive glycaemic control.20 A transient hyperopic shift occurred in all 28 participants with a reduction in blood glucose levels, with a minimum change of 0.50 D and a mean change in refraction of 1.47±0.87 D.
These findings were confirmed by a clinical study that reported transient variation in refractive status in diabetic individuals, although no clear trend in either a myopic or hyperopic direction was observed.21
Further evidence was provided by a study that investigated the effect of acute hyperglycaemia on retinal thickness and refraction, which reported that a small hyperopic shift can occur when acute hyperglycaemia was induced,22 which is in contrast to those studies reporting a hyperopic shift during intensive glycaemic control. However, this effect was observed in only one study participant and the authors concluded that ocular refraction was not affected by hyperglycaemia.
Lin and colleagues, in a small case series, also reported transient hyperopia due to intensive glucose reduction.4
Another paper considered and reviewed aspects around the crystalline lens in relation to blood glucose levels.23 The authors discuss refractive index gradients within the lens and applied mathematical modelling to determine whether and how such gradients impact on refraction. In their paper, they report that there is no simple linear correlation between blood glucose and refraction, at least in relation to short-term changes over several weeks. Even though the authors discuss recent work, which suggests that starting therapy to control hyperglycaemia leads to a hyperopic shift within a few days to weeks, followed by a gradual return to baseline over several weeks to months, they conclude that refractive changes occur relatively slowly (several weeks) and suggest that the transient nature implies that two mechanisms are involved. The absence of axial changes or curvature changes of ocular components suggests that changes in refractive index indeed play a role. In summary, the important finding was that it appears possible to account for any observed hyperopic shift after initiation of therapy for hyperglycaemia and the subsequent recovery based on changes in the distribution of refractive index within the lens.23
Huntjens, Charman and colleagues carried out a study to investigate how short term changes in blood glucose affect refractive components in individuals with type 1 and type 2 DM.24 For this clinical study, 41 long-term diabetic and 20 non-diabetic (control) participants were recruited and data were collected throughout the day at broadly two-hourly intervals between 8.00 and 20.00 hours. Various clinical measurements including objective refraction, aberrations, anterior chamber depth, lens thickness and corneal thickness were collected, one eye of each participant was randomly selected for statistical analysis. The study showed that short-term fluctuations of blood glucose levels did not cause acute changes in refractive error, aberrations, or anterior biometric parameters.24
In a recent ex-vivo study, a bovine lens model was used to assess optical changes in hyperglycaemia as well as in response to reductions in hyperglycaemia (back to normal glucose levels, simulating treatment onset).25 Back vertex focusing distance and equatorial lens diameter were measured. From these data back vertex focal length and longitudinal spherical aberration were derived. A statistically non-significant trend towards myopia with increasing hyperglycaemia was observed. Similarly, a hyperopic shift was noted for changes from hyperglycaemia to normal glucose levels, which was also not statistically significant. Overall, the results suggest that there is no consistent crystalline lens induced refractive change following exposure to hyperglycaemia for periods of up to 5 days.25
Zhu and colleagues assessed the frequency of under-corrected refractive error among diabetic individuals in Shanghai, China.26 Data were collected through a community-based study that involved a survey of 649 people aged 60 years and older living with DM. A range of clinical measurements was carried out including refraction, best-corrected visual acuity, tonometry, slit-lamp biomicroscopy and fundus photography. A key finding of the study was the observation that undercorrected refractive error occurred in approximately 17% of the participants, thus providing an indirect indication of a possible link between DM and refractive status.26 Similar studies have been carried out, for example in India, where a high prevalence of refractive error was observed in diabetic individuals (type 2) aged 40 years and older.27
In another study carried out in China, Song et al. set out to determine the prevalence of refractive error and the association with glycaemic control in adults living with type 2 DM.28 A total of 839 participants were included in the analysis, 96% of whom presented with some form of refractive error. Haemoglobin A1C (HbA1C) levels were found to be associated with refractive status in that myopic individuals had higher and hyperopic individuals lower levels of HbA1C. Overall, this may provide some, albeit not necessarily robust, evidence of a potential link between glycaemic control and refractive error and the authors recommend further longitudinal research to assess the relationship between glycaemic control and refractive status over time.28
Discussion and Conclusions
The impact of poor glycaemic control and hyperglycaemia on the refractive status in humans has been a topic of interest to the clinical and research community for at least a century. Yet, the studies reviewed here provide examples of the diversity of study designs, research methods, outcome measures used and resultant findings. For example, study designs included observational case series,18 4 cohort studies,19 clinical cross-sectional studies using human participants,4,20–22 population-based studies,26 ex-vivo animal studies25 and mathematical modelling studies.23 The variety of study designs and methodological approaches applied makes a direct comparison of the outcomes difficult. However, together these studies indicate that there is remaining uncertainty and that there is no consistent or robust association between glycaemic status, glycaemic control and refractive error, or changes in refraction.
However, there seems to be sufficient evidence to support the notion that short-term and reversible changes in refraction can occur in some individuals, for example in situations where blood glucose levels either drop or rise drastically. Similarly, the initiation of treatment to normalise blood glucose levels appears to cause a change in refraction, frequently in the form of a hyperopic shift. These refractive changes are not usually long-lasting and should thus not be considered in the long-term refractive management of patients.
The time course of the refractive changes varies, but the studies reviewed indicate reasonably strongly that a stabilisation of refractive status can be achieved within weeks of initiating or adjusting normoglycaemic treatments. The exact timeline needs to be determined for each individual patient. To determine the time point of refractive stability, regular follow-up appointments to assess the refractive status should be arranged, e.g. at 2-4 weekly review intervals, but always dependent on the individual situation of the patient. These follow-up appointments would allow for any trends such as any hyperopic shifts to be measured. Ophthalmic appointments could be coordinated in line with general medical appointments, potentially facilitating comparative assessments of blood glucose and refraction monitoring (where feasible).
Even though it is not possible to provide a definitive guide on when exactly refractive stability will be achieved, clinicians should ideally monitor patients in whom glycaemic control has been initiated or is being considerably adjusted and wait for a stabilisation of blood glucose levels before considering the prescription of spectacle lenses. Communicating the need to wait to patients is critical to ensure patients are fully aware of the reasons their visual problems are not being managed with spectacles immediately. The monitoring of blood glucose and its stabilisation are typically overseen by general medical practitioners or DM specialists. If patients present with visual complains and unmet refractive needs, there is an opportunity for interdisciplinary, collaborative care of such patients, involving close communication between optometrist and medical practitioner as well as the exploration of short-term solutions for the patient in order to help them achieving best possible visual outcomes while their glucose levels are being brought under control and spectacle prescribing can commence.
Conflict of interest
The author has no proprietary interest in any of the materials and methods mentioned in the article.
Acknowledgements
The author would like to thank Dr. Claudia Geue for critical review of and comments on the manuscript.
Ethics statement
This article reviewed the published literature and did not use any patient or other identifiable data. Ethical approval was thus not required.
COE Multiple Choice Questionnaire
The publication "The impact of diabetes mellitus and hyperglycaemia on the refractive status of the eye" has been approved as a COE continuing education article by the German Quality Association for Optometric Services (GOL). The deadline to answer the questions is 1 January 2023. Only one answer per question is correct. Successful completion requires answering four of the six questions.
You can take the continuing education exam while logged in.
Users who are not yet logged in can register for ocl-online free of charge here.
