Non-Arteritic Anterior Ischemic Optic Neuropathy
Purpose: The case report describes the clinical approach to a patient with optic neuropathy in an optometric practice by simple yet valid methods which can be used auxiliary to standard procedures.
Material and Methods: Funduscopy was performed via Volk 90D lens and slitlamp. Functional tests, such as visual acuity, pupil reactions, visual field assessment, colour vision, red saturation test, Pulfrich phenomenon and pupil cycle time test, were employed for their differential diagnosis value.
Results: The visual acuity and central colour vision were normal; the other tests revealed abnormal state of the patient‘s right eye or visual pathway. Ophthalmoscopy revealed a disc oedema in one half of the optic nerve head. After urgent referral with tentative diagnosis of optic neuropathy of unknown aetiology, a diagnosis of non-arteritic anterior ischemic optic neuropathy (NA-AION) was made at the eye hospital.
Conclusion: In patients with sudden onset of monocular visual disturbances where no obvious lesions in visual axis are found various types of optic neuropathies are a frequent finding. Some of these conditions include systemic diseases, such as multiple sclerosis, and some can be associated to life threatening diseases, such as giant cell arteritis.
Introduction
A 48-year-old female presented with a complaint of blurry vision in the inferior nasal portion of her right eye for several days. The patient reported that her ocular and medical history up to that point were unremarkable and her current systemic health was deemed to be good. Besides visual symptoms, she had no headache, no dizziness and reported no recent head trauma. Her refractive status was noted for mild myopia (ca. -0.50 D in both eyes) and visual acuity was good for as long as she can remember.
A sudden onset of monocular visual symptoms without any pain, visible inflammation or trauma indicates to retinal,1,2 vascular3 or neurological4 origin. A conscientious practitioner in primary eye care practice should be able to use appropriate examination techniques to determine the nature of the condition, judge the stage of urgency and refer the patient accordingly. In many practices, however, the diagnostic infrastructure is limited and optometrists are thus left with simple examination techniques. In the case presentation, we highlight some simple yet valid methods which can be used in addition to standard procedures.
Material and Methods
Visual functions were assessed with standard optometric methods: LCD chart for visual acuity (VA), phoropter, slitlamp with 90 D Volk lens; pseudoisochromatic plates (Ishihara) for colour vision testing. A special quadrant red desaturation test5 by Smart Optometry was used to assess colour desaturation. Further, the Pulfrich effect test6 and pupil cycle test (PCT)7 were performed. Additionally, we introduced an infrared pupilloscopy test performed with the meibography module at the slitlamp (Topcon SL-D 701 with DC-4 camera and BG-5 infrared illumination for meibography).
Results
A. Results of clinical procedures
The optometric status at the time of the first exam:
- Best corrected visual acuity (BCVA): OU 1,2 (low myopia)
- Colour vision (Ishihara): OD 12/12; OS 12/12
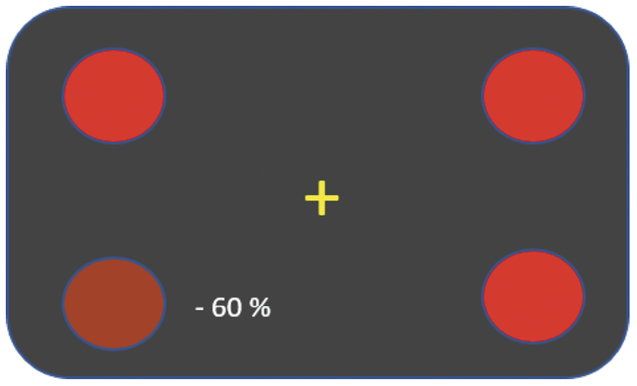
- Red saturation in quadrants (Smart Optometry app, Figure 1): OD desaturation by 60% in the lower nasal quadrant; OS saturation equal in all quadrants.
- Swinging flashlight test: OD moderate relative afferent pupillary defect (RAPD)
- Infrared (IR) pupilloscopy: OD afferent pupillary defect
- Pulfrich Phenomena positive: OD visual pathway conduction defect
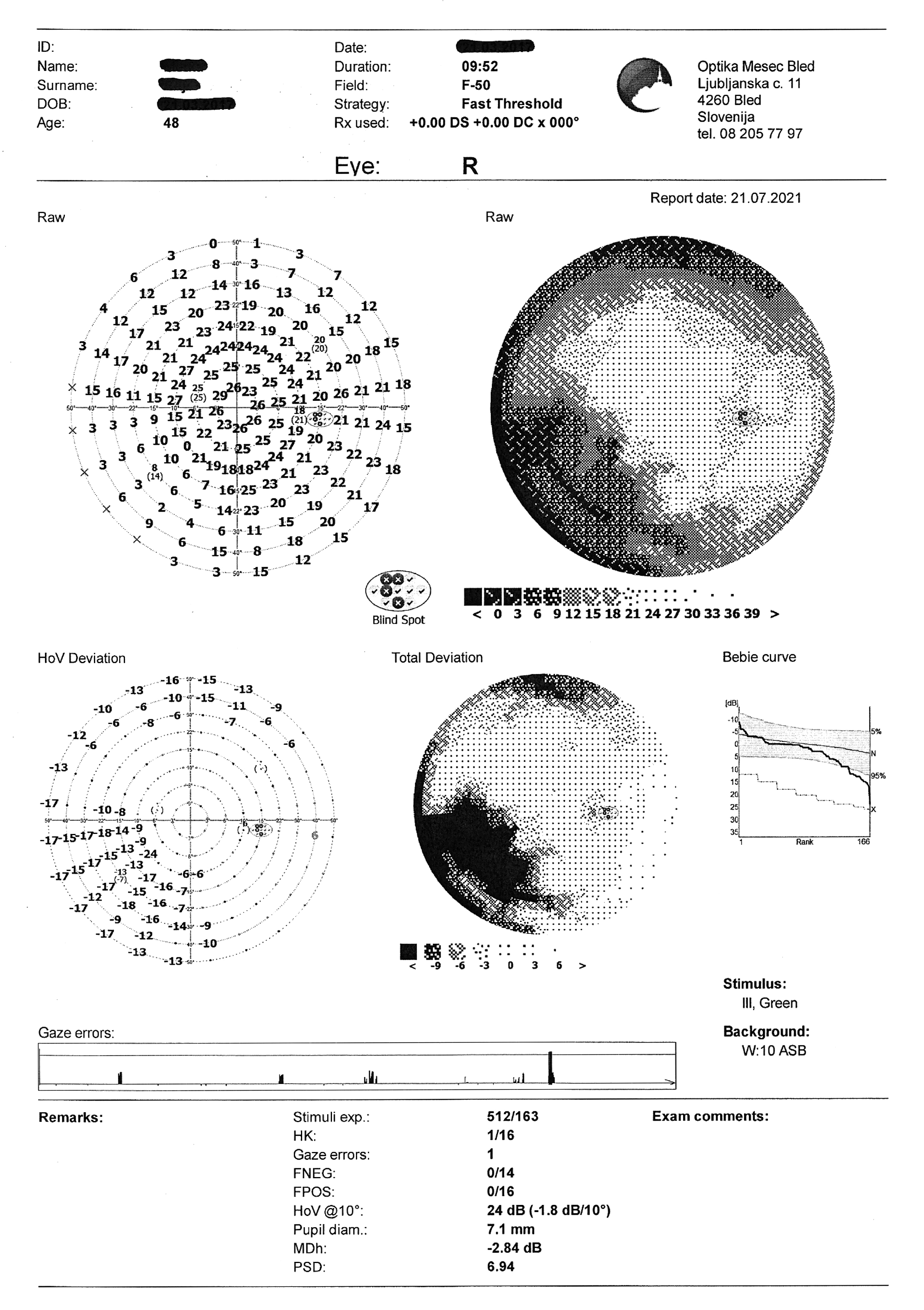
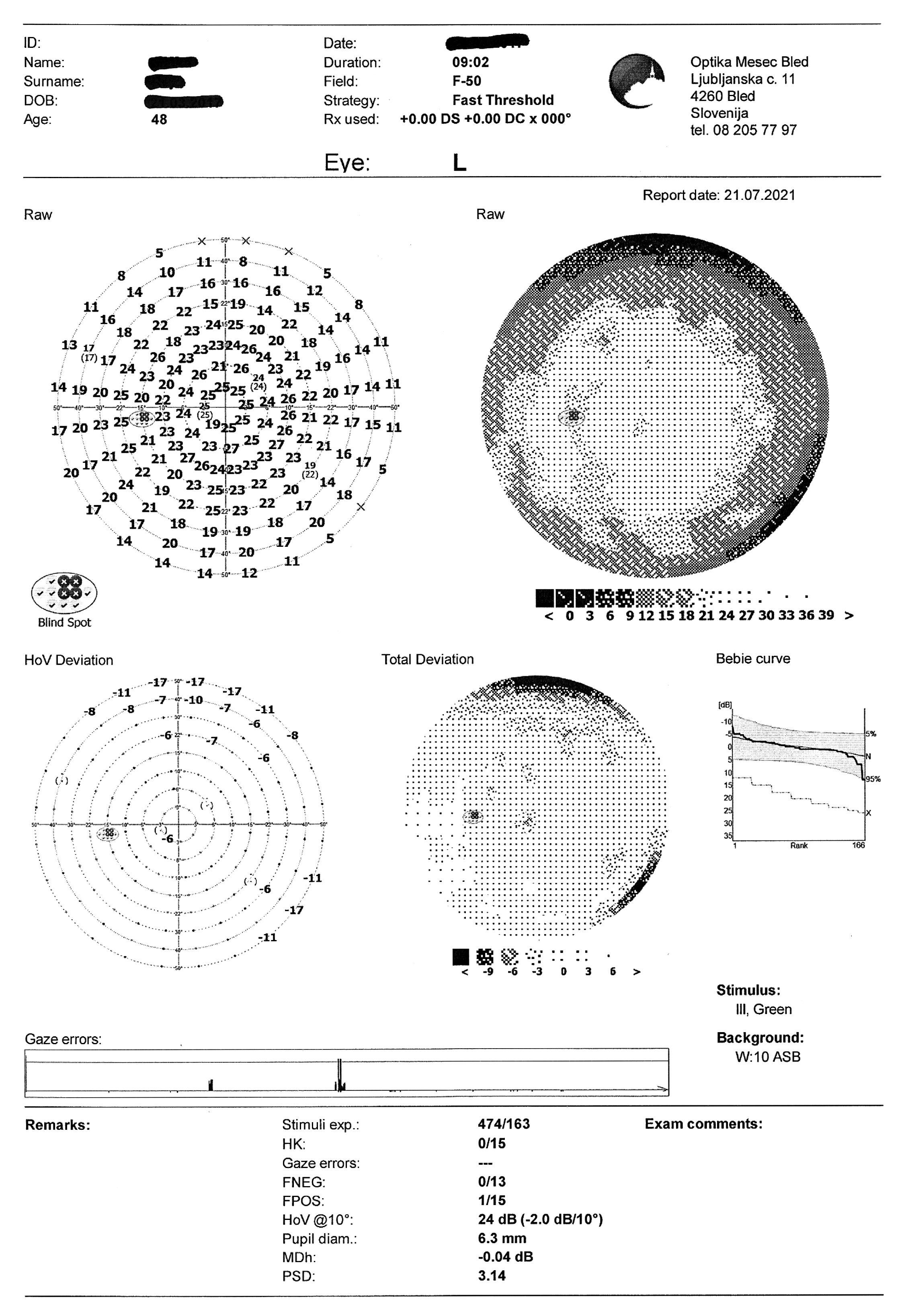
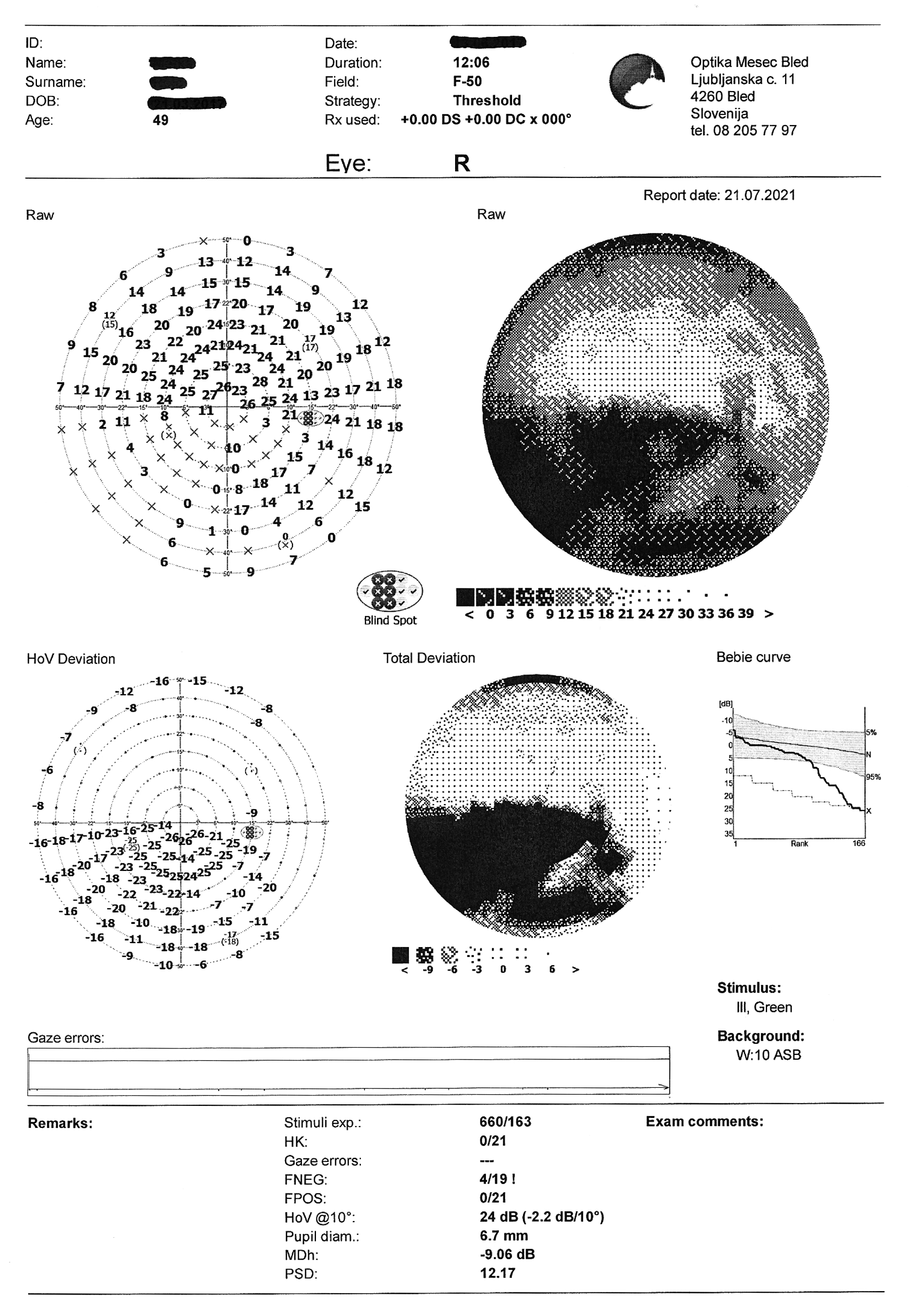
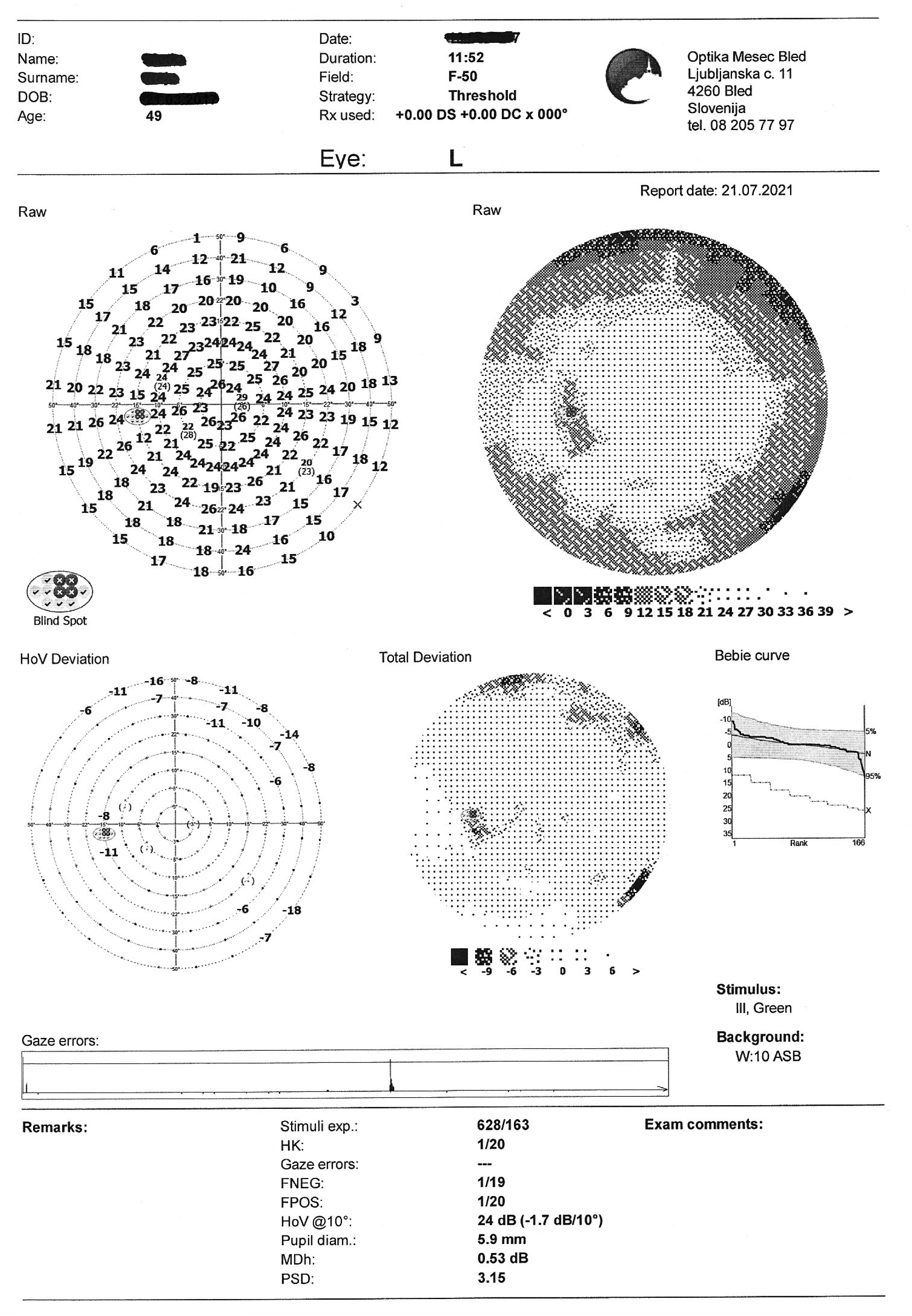
- Visual field test: OD partial inferior nasal visual defect
- Slitlamp examination: OU clear optical media; OD optic nerve swelling superior sector; OS unremarkable
Clinical test procedures
Red saturation in quadrants was quantified by the Smart Optometry app. The patient impression for OD shows -60 % saturation. In reality, the saturation in quadrants is continuously adjusted to establish equal subjective perception. Here the lower nasal dot was actually weighted + 60 % (Figure 1).
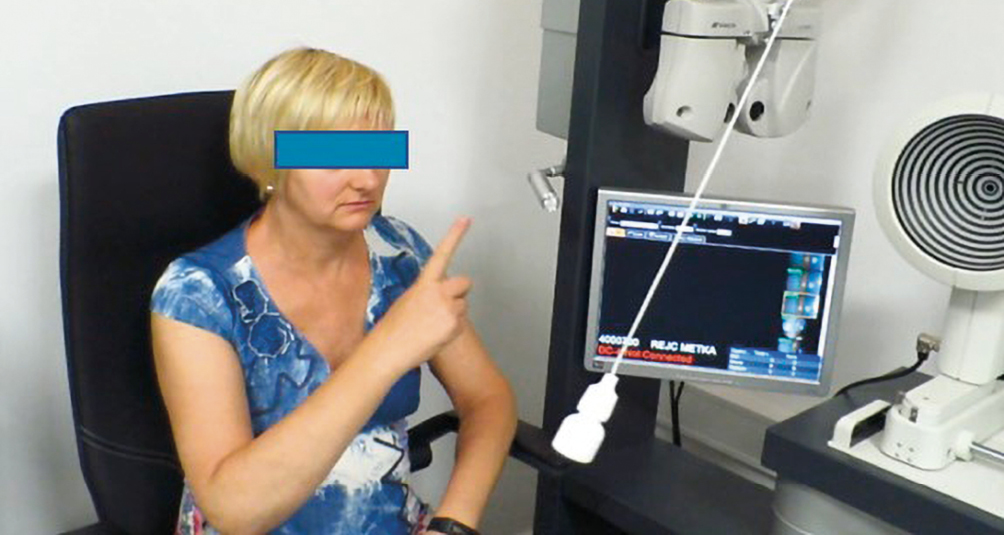
Infrared pupilloscopy was performed with the meibography module at the slitlamp (Figure 2). The camera with IR stays at the same eye whilst the flashlight is moved from one eye to the other. The procedure can be performed in a totally darkened room. Direct and indirect responses of the same pupil are observed on the screen, thus the afferent defect becomes obvious.
Presence of the Pulfrich phenomenon was assessed using a white pendulum (Figure 3). The patient perceived the
apparent circling of the pendulum. Disturbances in visual pathway conductivity cause the object in motion to be perceived disparately in relation to the other eye. Thus the patient has an impression of a stereoscopic image – the linearly swinging pendulum seems to circle in an elliptical pattern and appears closer to the patient when the direction of motion is from the sound eye to the diseased one.

Visual field test on static automated perimetry (Optopol PTS 920, F-50, fast threshold) revealed a partial inferior nasal visual field defect of the right eye. The superior depression in the OD and the superior and nasal depression in the OS are artifacts caused by eyebrow and nose (Figure 4).
In the superior part of the optic disc of the OD, a sectorial oedema could be seen. The disc of the OS is without remarks (Figure 5).
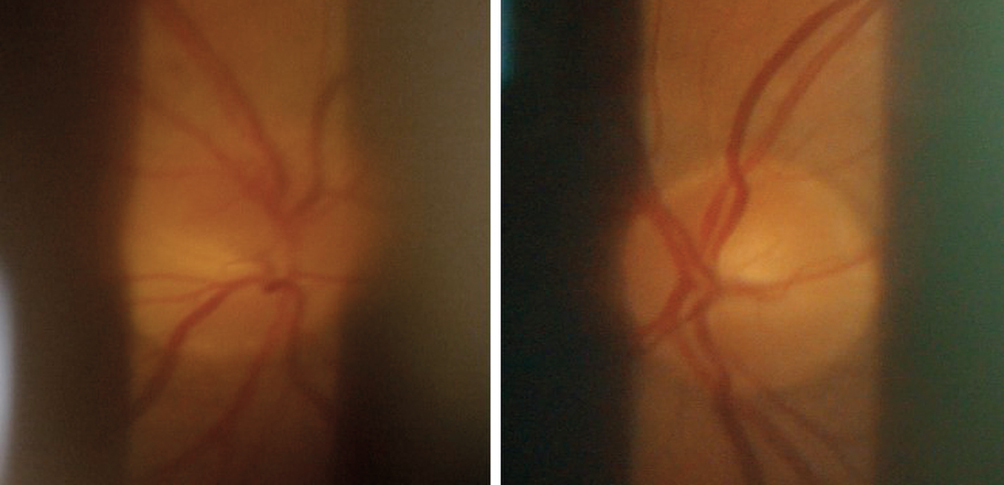
B. Tentative diagnosis
The findings clearly suggest a congestion in the optic nerve head. The tentative diagnosis was optic neuropathy of unknown aetiology. The patient was urgently referred to an ophthalmologist, who then referred her further to the university eye hospital. The final diagnosis was non-arteritic anterior ischemic optic neuropathy (NA-AION) in the right eye. The trigger factor or associated disease was not found. However, one year later, an ultrasound chest exam revealed a patent foramen ovale (PFO) in the heart causing micro-infarctions throughout the entire brain.8 The clinician commented that the condition was probably in place for several years. Besides visual symptoms, the patient remained asymptomatic.
After one month, the patient returned for follow-up assessment of visual functions, in parallel to her ophthalmological exams at the medical centre. At that time, she felt that disturbances of vision had slightly worsened. The BCVA of the right eye revealed a drop to 0,7 vision. Another visual field test was performed which revealed a dense inferior altitudinal defect (Figure 6).
During the follow-up visit, pupil cycle time (PCT)9,10 was also assessed. As suspected, the PCT in the OD was sluggish and did not achieve the normal 1 s-1 pulsation rate. The PCT in the OS was normal.
One and half year after the onset of NAION, the patient’s visual function remained stable, but the near vision with varifocal lenses was troublesome for her. To alleviate symptoms, new varifocals with bilateral yoked base down prisms (3 cm/m BD OU) were prescribed. Thus, the visual field was shifted into the remaining portion of functional retinal area. The adaptation was instant and very successful.
Discussion
Although the patient presented with relatively mild symptoms, the condition of non-arteritic ischemic optic neuropathy was serious and caused half of the visual field in one eye to be permanently lost. Since this was her dominant eye, the patient was unable to perform her duties as a dental technician and was on sick leave for one year. Later on, she had to find another workplace with less demanding visual tasks.
At 48 years of age, the patient was relatively young for the diagnosis given. AIONs normally occur at later stages of life due to infarctions of the laminar or retrolaminar portion of the optic nerve head that is supplied by the short posterior ciliary arteries. Generally, ischemic optic neuropathies are divided into arteritic11 (A-AION) and non-arteritic (NA-AION).12,13 The A-AION is associated with giant cell arteritis (GCA)/temporal arteritis (TA)14 which can be life-threatening conditions. The hallmark of A-AION is vascular inflammation, which is classically detected through an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels.15 Temporal artery biopsy is often performed to confirm GCA. NA-AION cases are more common and usually affect half of the visual field in an altitudinal fashion. The most common visual field defect reported was in the inferonasal portion.16 The visual field defects usually remain permanent and only rarely do they improve over time; complete resolution has not been reported in literature so far.17 AIONs are often associated with various systemic conditions, such as arterial hypertension, diabetes mellitus, gastrointestinal cancer, ischemic heart disease, cerebrovascular disease and chronic obstructive pulmonary disease.18
Conclusion
In the present case, the patient suffered substantial functional vision loss due to NA-AION which could not be prevented. Being aware of possibly life-threatening variants of AION, practitioners need to take a detailed patient history and be familiar with assessment techniques. Besides some simple standard procedures, infrared pupilloscopy with a meibography module on the slitlamp can also be utilized. This first time presented technique has good potential in detecting RAPDs in dark irises and can also be a useful tool in detecting bilateral RAPDs. In rehabilitation of altitudinal visual field defects, yoked prisms can be used, similar as in their better-known application in homonymous hemianopsias.
Permission
Patient agreed to the images being used for publication purposes.
Conflict of interest
The author served as an advisor to the Smart Optometry app development and introduced the interactive red desaturation test design. However, he has no financial interest in this particular project.