Possible ocular effects of anti-diabetes medications, and their relevance to contact lens wear
Purpose: To asses what information on ocular effects of antidiabetes medications are provided in medicines prescribing information, and whether or not any current guidelines actually mention contact lens wear.
Material and Methods: Medicines directories and patient information leaflets (PILs) for 28 diabetes medicines (insulin and various oral hypoglycaemic drugs) were searched for any information on possible ocular effects. A PubMed search was also undertaken on the medicines in relation to ocular side effects.
Results: Almost all information sources examined included notes that a change in vision after taking anti-diabetes medications could indicate inadequate glucose control. The use of all diabetic medications can result in mild to moderate allergic reactions that could involve the conjunctiva, eyelids and eyelashes. A possible adverse effect of use of older oral hypoglycaemics (such as the sulfonylureas and acarbose) is a warning to patients of possible ‘yellowing of the eyes’ and of the skin. In the vast majority of published studies identified, almost no information has been provided on the oral antidiabetes medications being used so it is essentially unknown if diabetic medications per se can have adverse effects on the cornea and conjunctiva. However, the information search (in both medical directories, PILs) revealed nothing in relation to contact lens wear and/or that there should be any obvious caution in prescribing contact lenses to diabetic patients.
Conclusion: Information indicating that contact lens wear is potentially problematic is not obviously included in medicines directories, so contact lens wear can offer a viable option for vision correction in diabetics.
Introduction
Diabetes mellitus is perhaps the most avoidable and controllable sight threatening disease of the eye, and it has long been recognized that there can also be effects on the anterior segment of the eye.1,2 This has been re-emphasized very recently,3 noting that both the American Optometric Association and also the American Academy of Ophthalmology recommend that when patients are diagnosed with type 2 diabetes they should be provided with a full eye examination and reassessments every year, while those diagnosed with type 1 diabetes should be assessed within 5 years and then annually thereafter. High street optometrists (i.e. in community practice) are very well placed to provide such eye examinations and specifically to examine the ocular surface of their diabetic patients, especially those who are contact lens wearers or considering this mode of vision correction. However, equally strongly, a case can be made that the relationship between diabetes and the occurrence of some form of dry eye giving rise to symptoms is far from clear, with substantial differences in reported clinical tests and expression of opinions being a characteristic of the literature over the last 30 years. This especially applies to contact lens wear in diabetics.
An interesting outcome of a small practitioner survey in the mid-1980s, noted that ‘Fifty percent of those who experienced problems with the wear of hard contact lenses had diabetic relatives’;4 the type of contact lenses used then would have been remarkably different from those predominantly in use nowadays. Similarly, other earlier perspectives need to be considered within the context of modern soft or rigid contact lens materials. Clinical and animal studies were undertaken in the early 1990’s to assess the effects of fitting soft contact lenses to diabetic individuals.5 From these, it was concluded ‘a decreased corneal edema recovery ability’…. ‘suggests that care should be taken when prescribing contact lens use in these people’.5 The decreased corneal edema recovery refers to a slower rate of corneal thickness return to ‘normal’ daytime levels of the patient with type 1 diabetes. Similarly, in assessment of patients in the UK in the mid-1990s, eight were advised that ‘they could not wear contact lenses because of their diabetes’.6 Notwithstanding, in another practitioner-based survey carried out in Canada in the early 1990’s, patients were asked whether or not they thought they had ‘dry eye’ essentially on a Yes/No basis.7 The same patients were also asked to indicate which medications they were taking and if they responded yes to ‘medications for diabetes’, then the incidence of reporting ‘Yes’ to the dry eye question was 1.35 X higher as compared to those who did not report use of diabetes medications. This very simplistic assessment can be criticized (although it was the first large scale questionnaire of its type with 13,517 respondents), but it be can noted that this type of un-weighted odds ratio for dry eye being experienced was 1.86 if the respondents of any age had indicated ‘Yes’ for ‘oral contraceptives’, and 1.69 for older female patients using hormone replacement therapies (HRT). It was higher still (at 2.03) if the patient reported use of ‘diuretics (medicines for kidneys)’. There is high relevance of the latter two medication groups to older patients with type 2 diabetes. As a closing comment on unacceptable central corneal thickness (CCT) increases associated with systemic conditions or contact lens wear, the issue of whether actual measurements of CCT should be made. From a comprehensive review of the literature published over a 30-year period, it was concluded that ‘Routine contact lens wear and diseases such as diabetes seem unlikely to produce changes in CCT of a magnitude that would justify pachometry as a monitoring method beyond routine slit-lamp evaluation’.8 Irrespective of a perception of avoidable risk of developing unacceptable corneal thickness increases by wearing contact lenses if diabetic, and potentially experiencing excessive ocular discomfort, there are uncertainties, and the aim of this review is to provide an update on the topic.
As both contact lens properties as well as the medications for diabetes mellitus have changed substantially since the 1990’s, the broad goals of this review will be to provide the practitioner with an up-to-date summary / overview of anti-diabetes medications, especially the oral hypoglycaemics. This is something that is essentially absent from the peer-reviewed eye-related literature. In addition, a review will be undertaken of what information on any ocular effects of these medications are provided in pharmaceutical directories and patient information leaflets, as well as to assess whether or not any of these possible effects have relevance to contact lens wear in diabetics and whether or not contact lens wear is mentioned in any current guidelines on use of diabetes medications. With these goals completed, it is hoped that this review will provide an overall current perspective on the safety of carefully monitored contact lens wear in the diabetic patient, and what that monitoring could involve in a routine optometric practice with a slitlamp.
Material and Methods
Medicines and pharmaceutical directories (e.g. British National Formulary or Monthly Index of Medical Specialities, in the UK) and patient information resources for 28 anti-diabetes medicines (insulin and various oral hypoglycaemic drugs), were manually searched for any information on possible ocular effects. The sections labelled precautions, warnings, side effects, adverse effects and drug interactions were specially examined in the medicines directories. For patient information leaflets (PIL) that are provided with the medicines on prescription and which are now usually available on line and regularly updated, the sections on use, sub-sections titled ‘warnings, or ‘side effects’ were especially scrutinized for any relevant notes on vision. Finally, PubMed (www.ncbi.nlm.nih.gov/pubmed) was searched under all 28 drugs AND separate keywords including ‘eye’, ‘vision’, ‘cornea’, ‘conjunctiva’, ‘sclera’ and ‘contact lens’ to try to identify any peer-reviewed publications (including any case reports) that reported on the possible ocular effects of these drugs.
Results
Medications for diabetes
Diabetes is medically treated only with insulin if a patient (usually younger) is diagnosed with insulin-dependent diabetes (i.e. type 1 diabetes). The insulin is administered by injection with a variety of devices beyond a simple hypodermic needle now available. Type 2 (initially non-insulin-dependent) diabetes is more commonly diagnosed in older patients (usually from 40 years and older), often associated with confounding health conditions such as obesity and high blood pressure. This condition is initially medically treated with a combination of one or more oral medications (known as oral hypoglycaemic drugs, that promote the pancreatic function to produce at least some insulin) but ultimately may need to be managed with a combination of these drugs (a few of which are administered by injection) as well as insulin (by injection)
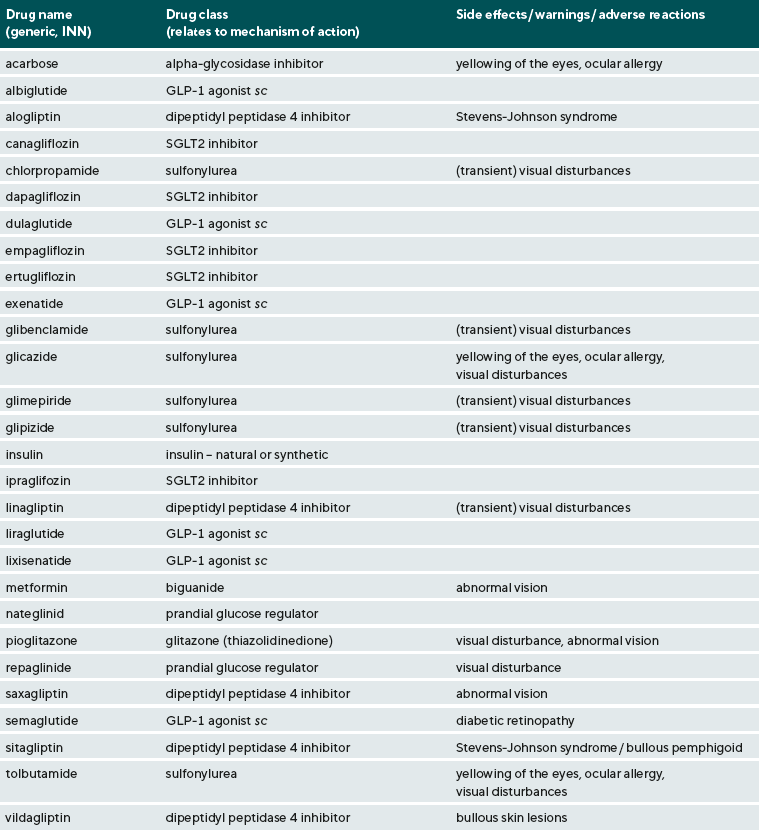
There are now many options available to the general medical practitioner for treating diabetes. An up-to-date list of these, in alphabetical order, is given in Table 1. Other than insulin, other injectable drugs (identified with an ‘sc’ next to the name in the table) include a group known as GLP-1 agonists (glucagon-like peptide-1 agonists, with glucagon being one aspect of pancreatic insulin secretion control) and currently may include albiglutide, dulaglutide, liraglutide, lixisenatide and semaglutide (with availability depending on country and appropriate medicines use legislation). All other diabetes medications are taken orally, with some indication of relationship to meals, with the initial dosing being anywhere between once-a-day to three times-a-day, and then adjusted down to once or twice daily if the response is adequate. The earliest oral hypoglycaemics included drugs known as the ‘sulfonylurea’ class, which may be represented by chlorpropamide, glibenclamide (perhaps also listed as glybenclamide), glicazide, glimepiride, glipizide, and tolbutamide. Several of these may only be available as generics and not as branded medicines. Another older type of oral hypoglycaemic drugs is the alpha-glycosidase inhibitor, introduced over 50 years ago, and represented by acarbose. The longevity of use of these older medications is a testament to their efficacy, but they did have some side effects perhaps most-notably an allergic reaction to ‘sulpha’ drugs. However, with the ever-more specific modern-day medicinal approach to diabetes to try to control blood sugar levels to within very close pre-defined limits for a particular patient, the biguanide represented by metformin is likely a first choice for oral hypoglycaemics; This can be used alone or in combination with other drugs such as dipeptidyl peptidase 4 inhibitors (represented by alogliptin, linagliptin, saxagliptin, sitagliptin, and vildagliptin), SGLT2 inhibitors (sodium glucose transporter 2 inhibitors, represented by canagliflozin , dapagliflozin, empagliflozin, ertugliflozin and ipraglifozin), and a group of drugs referred to as glitazones or thiazolidinediones (now likely only represented by pioglitazone). The newest option, also for possible administration with metformin, are drugs referred to as ‘prandial glucose regulators’ (represented by nateglinid and repaglinide). There may be a few others, but Table 1 represents a summary of the most commonly prescribed medications available in the UK and Europe (and is the most complete list as compared to any others that could be found in any peer-reviewed publication).
As indicated earlier, one of the main goals for the present review was to assess what information on any ocular effects of these medications might be generally provided. It was of special interest to assess whether any mention of contact lens wear could be found in either the general medical / pharmaceutical directories used by medical professionals and especially the patient information leaflets. It can be noted that the search revealed nothing in relation to contact lens wear, nor was there an indication that there should be any obvious caution in prescribing contact lenses to such patients. This outcome was obtained despite considering every diabetic medicine and using a variety of sources. To put this outcome in perspective, it is commonplace for mention of contact lens wear in even the briefest of directory entries or PILs for oral contraceptives and HRT. This is usually as a note something like ‘contact lens intolerance’ can develop, although no listing could be found that indicated that there should be any obvious caution in prescribing contact lenses to such patients.
Virtually all information sources examined included notes of various types, especially under ‘warnings’ that a change in vision after taking anti-diabetes medications could indicate inadequate glucose control (e.g. as associated with onset of hypoglycaemia) and that caution should be exercised in using heavy machinery or driving if vision was blurred. It was also commonplace for there to be notes, especially in the PILs, that vision changes were usually temporary and were associated with the bodily adjustment to medicines-imposed control of glucose. Various terms are used such as ‘abnormal vision’, ‘blurred vision’ or ‘doubling of vision’. Notwithstanding, for a few of the medications, such effects appear to be considered as more substantial (and even commonplace) to warrant a warning or adverse effects note in pharmaceutical directories of ‘visual disturbances’, ‘transient visual disturbances’ or ‘abnormal vision’. These can be found for the sulfonylurea drugs, the dipeptidyl peptidase 4 inhibitors, and the biguanide and glitazone group drugs. Such changes can be expected to be fully reversible, especially as the drug dosage is adjusted to be just right for a particular patient. Optometric practitioners are well placed to advise their diabetic patients to see their medical practitioner if vision changes are occurring and may even enquire as to whether the patient is regularly checking their blood glucose level.
For only one of the newer drugs, semaglutide, there is currently a much more specific warning associated with the risk of progressive vision deterioration, i.e. worsening of the diabetic retinopathy. When medical therapy of diabetes is introduced, it is important that there is no worsening of the condition, and this will be routinely assessed in initial clinic trials of these drugs as ‘outcome measures’. Some initial worsening, including in retinal status (e.g. if retinopathy develops or progresses further), can occur and the drug dosages usually need to be adjusted to achieve better glucose control. While it might seem a rather vague indicator to a specialist optometric practitioner, the assessment of the worsening or improvement of the eye in diabetics in terms of whether there were ‘eye disorders’ is considered to provide useful comparisons between medical therapies. So, for example, using rather more complex odds ratio calculations, it has recently been noted that the incidence of ‘eye disorders’ was 1.15 higher in patient groups treated with sulfonylureas compared to dipeptidyl dipeptidase 4 inhibitors, but only 1.05 as compared to patients on thiazolinediones (i.e. pioglitazone).9 A similar outcome (i.e. very slightly better retinopathy control with newer drugs as compared to sulfonylureas) was obtained in consideration of prescribing any of the GLP-1 agonists with the exception of semaglutide.10 Overall, while the adjunct use of GLP-1 agonists has been found to be beneficial in control of blood glucose levels with acceptable side effects and the expectation of offsetting of the ocular side effects of diabetes, the incidence of deterioration of retinopathy has been found to be higher in some studies on semaglutide. It has yet to be clearly established whether the deterioration of retinal function is related to inadequacy of other aspects of glucose control or whether the drug may exert some type of toxic effect on the retina. As there is uncertainty, a specific cautionary note is to be found in current medical information sources.
An adverse effect of use of oral hypoglycaemics and the anterior segment of the eye is the listing of ‘yellowing of the eyes’ or similar with PIL’s for the sulfonylureas and acarbose advising patients of possible ‘yellowing of the skin and whites of the eyes’ or even using the term jaundice. This would be ‘scleral jaundice’ associated with cholestatic liver disease, with discoloration or icteric appearance developing within a few weeks after starting therapy and can spontaneously regress. A contemporary source of adults treated with these drugs, with colour image for example, could not be found, but this is a side effect considered to be very important in the late 1950’s and early 1960’s and leading to failure of clinical trials of several oral hypoglycaemics.11 Whether or not this has occurred to any significant extent in recent years is unclear. Notwithstanding, since blood bilirubin levels (linked to liver function) might be linked to peripheral neuropathy in type 2 diabetes,12 it might be prudent to schedule extra slitlamp assessments including corneal staining and fluorescein tear break up time measures in such patients.
Warnings for the possible development of allergic reactions involving the mucous membranes of the eyes are noted for some drugs. Some of these reactions can be severe (i.e. bullous pemphigoid or Stevens-Johnson syndrome) being noted for the newer dipeptidyl peptidase 4 inhibitors and while only ‘ocular allergy’ is noted in medical directories consulted, PILs for some of the sulphonylureas (e.g glicazide) do note the more severe allergic reactions specifically. It should be noted that allergic reactions may also involve the eyelids and the eyelashes.
As a closing comment on the effects of medications for diabetes on the ocular surface, the issue of details of these medications needs to be addressed, especially for type 2 diabetics. Based on published literature relevant to diabetes and the anterior eye over a 30 year period, a case could be made that more information needs to be provided on these medications. Examples where information is provided appear to be rare. In an early study on type 2 diabetics, it was noted that one third were taking oral hypoglycaemics (‘orale Antidiabetika’), but no details of specific drugs were provided.2 The same applies to other reports where it has simply been noted that the patients studied were using ‘oral hypoglycaemics’, 13 ‘oral antidiabetic drugs’ referred to as OADs,14 or ‘oral glucose-lowering drugs’, OGLDs.15 In a very recent study however, it was noted that the results of some fairly complex statistical analyses on a mixed group of type 1 and type 2 diabetic patients indicated not only that age was associated with a higher incidence in dry eye (in type 2 diabetics) but also that the use of metformin was possibly also linked to the higher number of patients reporting dry eye symptoms.16 Such an example indicates that this information (e.g. the names of the oral hypoglycaemic drugs being taken, along with details of duration of the type 2 diabetes would be a useful starting point) could be very useful to assess whether or not the cause of ocular symptoms or changes in tear film stability, for example, are due to the diabetes per se, or the medications being used. As noted earlier, inadequate control of blood sugar levels can lead to worsening of diabetic eye disease or result in some visual symptoms as blood glucose levels fluctuate and medicines selection and dosing need to be reviewed. The severity of any anterior eye changes could be simply linked to inadequate control of the diabetes. However, without details being routinely provided of medications being used for type 2 diabetes in peer-reviewed literature, it cannot be ascertained whether or not anterior eye changes are due to adverse effects of the type 2 diabetes medications on the cornea or tear film. If the outcome of this recent study can be confirmed, it could open up the possibility for medical practitioners, perhaps in liaison with the patient’s optometrist, to select an oral hypoglycaemic agent based on its effects on the anterior eye.
Contact lens wear in diabetes
As noted in the introduction, prior to a range of independent studies being carried out, some optometric practitioners either advised against contact lens wear,6 or that extra special care should be taken.5 The overall concern in the earlier considerations of this issue, although not explicitly stated in any peer-reviewed article that could be found, was that the diabetic patient wearing contact lenses could develop unacceptable levels of corneal oedema. This could be manifest as both pronounced epithelial oedema (haziness) as well as stromal swelling evidenced by the striae. Striae indicate compression lines (folds) in the posterior corneal stroma associated with a swelling of probably close to or slightly higher than 10 % above normal levels for that cornea. There is no absolute value because of the wide range of corneal thickness values (of around ± 20 % of an overall mean) that can be encountered in nominally normal adults, with around ± 2 % of any differences being accounted for by the use of different measuring (pachymetry / pachometry) systems.8 While the changes shown in Figure 1 were reversible within 2 h, an overall concern might be that if diabetics already had a slightly thicker cornea than normal,5 then the extent of swelling of the cornea associated with contact lens wear would be even greater than in non-diabetic individuals resulting in unacceptably persistent corneal oedema. However, it can be noted that in a cross-sectional observational study by optometrists of diabetics already wearing soft contact lenses (over an average period of 13 years), a few did present with striae indicating that these individuals were not obviously bothered by the oedema. It was concluded, however, that corneal thickness values in the group of diabetics studied were not substantially different from those found in lens-wearing non-diabetic subjects, i.e. unacceptable levels of corneal oedema were not generally observed.17
In carefully conducted challenge studies, diabetics have been fitted with very thick (0.3 mm) soft contact lenses with eyes closed for 2 to 3 h to assess just how substantial an artificially-induced corneal swelling might be. Contrary perhaps to expectations in the early 1990s, a notable aspect of the induced corneal is that, while notable, the averaged net increase in corneal thickness was less in the diabetics.18 This was observed in the early study by Herse and Hooker,5 and also reported from three other prospective studies,19-21 while a conclusion of no measurable difference in the induced was provided in two other study reports.22,23 Some of these investigations were on mixed groups of individuals with either type 1 or type 2 diabetics.19,21,23 A notable exception to the overall outcome from these seven studies, therefore, is that from reports of another earlier study on type 2 diabetics, including those with severe retinopathy, where it was concluded that the thick contact lens-induced oedema was higher in the diabetics;24,25 The corneal thickness was also higher in these type 2 diabetics compared to non-contact lens wearing eyes. A similar outcome has also been reported in other reports of corneal thickness in long term diabetic contact lens wearers.20,21,26,27
Overall, therefore, while contact lens wearing diabetics (of either type) may present with slightly higher corneal thickness values, these appear to be tolerated. In a more recent questionnaire-based study of 250 diabetics wearing daily-wear soft contact lenses for more than 1 year, no overall increase in complications were noted as compared to 250 individuals without diabetes.28 It needs to be noted, however, that reliance on symptoms may be problematic since patients can become used to certain levels of lens discomfort. For other symptoms of eye dryness, for example, it is also relevant that recent studies using questionnaires indicate that patient-reported symptoms may show no relationship to the duration of diabetes.29,30
The current USA-based ophthalmic guidelines do not preclude contact lens wear for patients with diabetes.3As noted earlier in the review of diabetic medications, especially for type 2 diabetes, a feature of the reports on contact lens wearing diabetics is the notable lack of information on their medication details, excepting the report by Skaff and colleagues.18 As concluded in a recent review, evidence based on prospective and retrospective studies clearly indicate that contact lenses can be a viable mode of refractive error correction for diabetic patients.32 Notwithstanding, before generally concluding that contact lens wear is a viable option for older type 2 diabetics, some further studies would be useful, particularly if the medication details are provided.
Conclusions
Overall, it is concluded that there are no notable reasons why diabetics with reasonably healthy eyes cannot wear contact lenses. The type of contact lens selected should logically be left to the practitioner since the extent of their experience with a certain type of lens (i.e. soft lenses, SiH lenses or RGP lenses) will facilitate their ability to detect any problems with lens wear in their patients. Optometric practitioners are well placed to emphasize the need for very good compliance with regards to CL handling and hygiene. Practitioners may choose to enquire of the blood glucose levels of their diabetic patients, to be better able to identify those with poorly controlled diabetes, especially type 2 diabetes. The key aspect of patient management is to try to ensure regular follow ups that should include a comprehensive slitlamp examination with fluorescein to assess the ocular surface and tear film.
Conflict of Interests
The author declares no conflicts of interest.