RGP contact lenses, retinal hole and choroidal nevi
Purpose: The case report describes the management of a retinal hole and two choroidal nevi during RGP contact lens fitting.
Material and Methods: During a comprehensive eye examination as part of a contact lens fitting, a retinal hole and two choroidal nevi were detected in the patient’s right eye by indirect ophthalmoscopy and Optomap ultra-widefield imaging (Optos Daytona Plus). The preoperative and postoperative findings and optometric/ophthalmological co-management are described.
Results: The retinal hole was completely closed by laser coagulation. The suspected diagnosis of two choroidal nevi was confirmed.
Conclusion: Myopia in particular is often associated with secondary retinal diseases. For this reason, regular retinal examinations are indicated in myopic patients. A comprehensive eye examination by an optometrist or ophthalmologist is normally recommended in the prescribtion of visual aids for those affected.
Introduction
A 70-year-old asymptomatic patient presented for the fitting of new contact lenses. She was wearing rigid contact lenses since the age of 41. The current lenses were two years old and poorly tolerated, therefore the patient wanted new ones. The patient‘s family and ocular history were unremarkable. Her overall health was good, and she took no medication.
Material and Methods
A comprehensive eye examination was performed as part of the contact-lens fitting.1,2,3 The anterior eye segment as well as the corneal topography were checked with a slit lamp and the Pentacam, while the posterior eye segment was examined using indirect ophthalmoscopy and a 90 D Volk lens as well as Optomap ultra-wide-angle imaging (Optos Daytona Plus). The intraocular preassure was determined with a Reichert CR7 non-contact tonometer.
Results
The visual acuity with correction was 0.8 in both eyes with a refraction of OD: −2.00 D cyl −1.50 D axis 3° and OS: −2.75 D cyl −2.25 D axis 10°. The central corneal radii were OD: 7.73 mm in 2° and 7.33 mm in 92° with a mean eccentricity of 0.30; and OS: 7.80 mm in 6° and 7.4 mm in 96° with a mean eccentricity of 0.38.
The examination of the anterior eye segment revealed no abnormalities except for small bilateral pingueculae (at 9:00 and 3:00) and four small, paracentral, subepithelial corneal opacities in the left eye. The anterior chamber was optically empty and deep on both sides, the pupils were round and reacted to light (MG: negative). The tear meniscus was
approximately 0.2 mm and the tear film was normal. The lens showed no significant clouding in either the right or left eye. The posterior eye segment of the right eye exhibited peripherally a small round retinal hole, two nevi (below and temporal to the optic disc) and a slight vitreous opacity (Fig. 1). Due to the lack of pigmentation, the round hole seemed to have developed recently. The two nevi exhibited a typical slate-grey colour. They did not appear elevated. The left eye had no retinal abnormalities. The cup/disc ratio was OD/OS 0.2. The optic nerve had in both eyes a normal colour and distinct margins; the macula was normal for the patient‘s age. The IOP was: OD 16.0 mmHg; OS 16.5 mmHg (11:00). Due to the retinal hole and the two choroidal nevi, the patient was promptly referred to an eye clinic. The eye clinic confirmed the suspected diagnoses of „choroidal nevi and round retinal hole“ in the right eye and treated the retinal hole with laser coagulation. 16 days later, the patient visited us again. The fixation of the retina along the edge of the hole by means of the laser coagulation was clearly visible. Pigmentation had not yet occurred (Fig. 2 and Fig. 3). The suspected diagnosis of choroidal nevi was also confirmed by the eye clinic and an annual check-up was recommended.
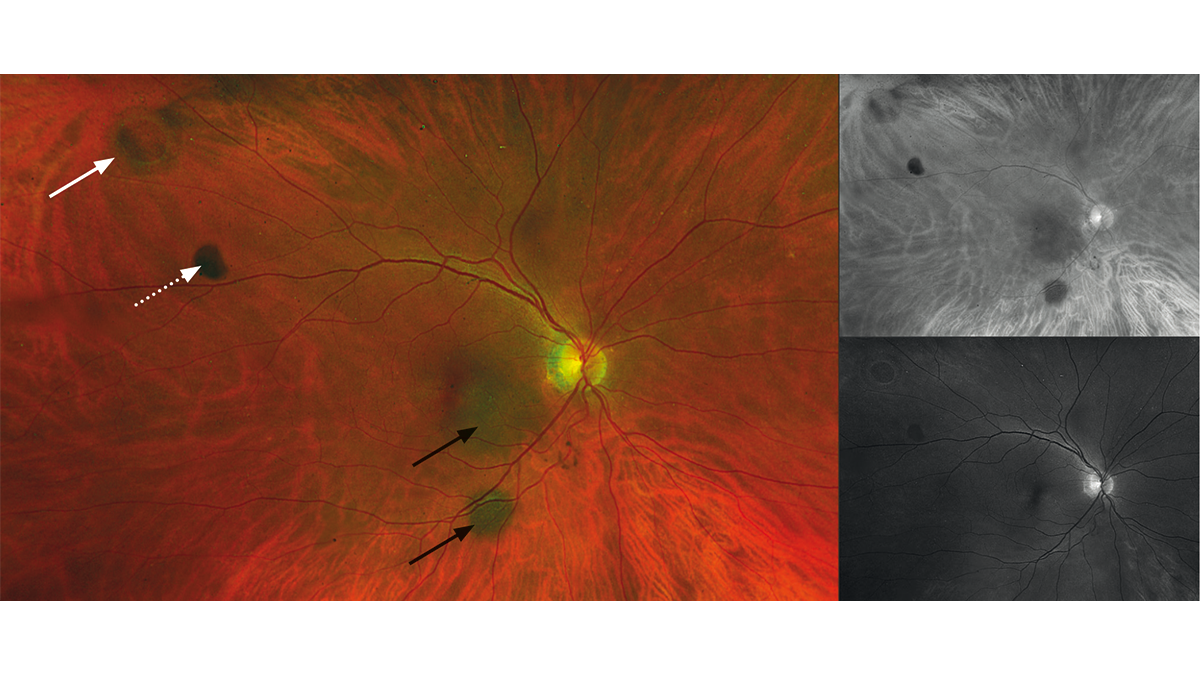
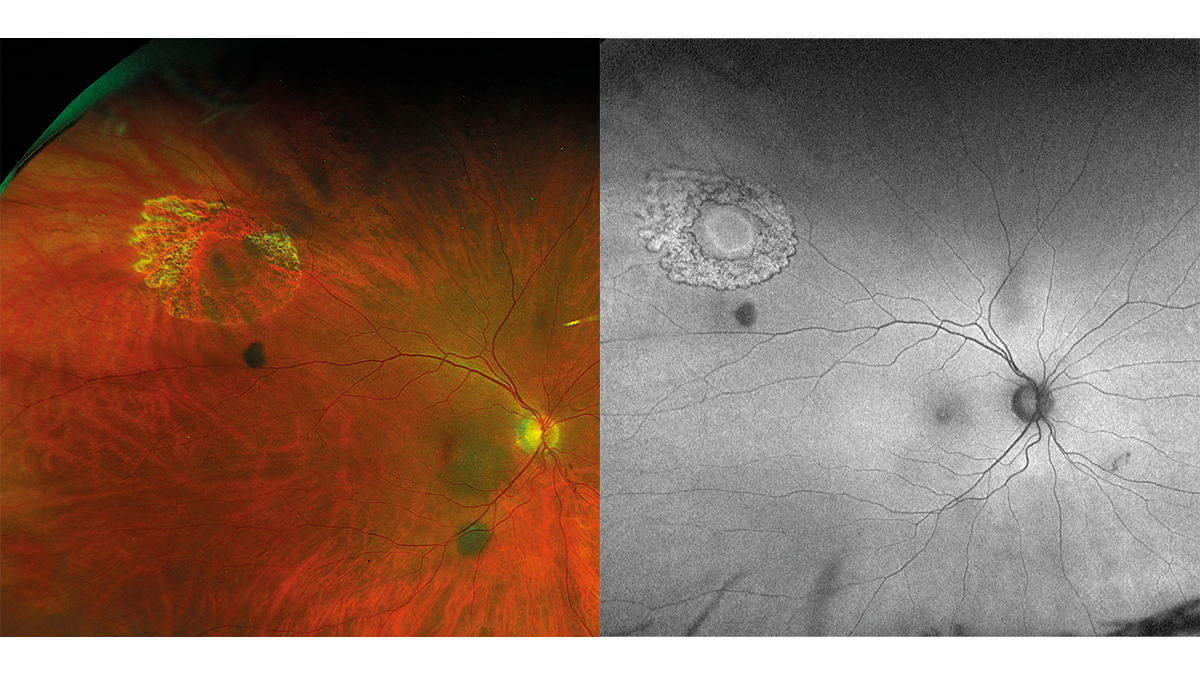
Discussion
Retinal holes
Small, atrophic, peripheral, round holes in the retina are one of the precursors of rhegmatogenous retinal detachment, with an incidence of 1.5 % to about 5 % in eye-healthy adults.4,5 Chen et al.6 found a prevalence of 4.4 % for peripheral retinal tears, holes or retinal detachment in Asian individuals aged 19 to 25 with high myopia and 2.6 % in emmetropes of the same age. Young et al. found a similar value with an incidence of 4.35 % for peripheral holes and tears in patients with high myopia prior to implantation of a Collamer lens (ICL).7 Retinal holes can be round, oval or U-shaped. They appear red due to the lack of retinal tissue, which attenuates the choroidal reflection.8 Basically, a retinal hole can be asymptomatic or associated with flashes, floaters, shadows or fog in affected patients. Since small peripheral retinal holes are usually asymptomatic, they are often discovered as a chance finding in the course of a comprehensive eye examination. Atrophic peripheral retinal holes are the result of focal degeneration of the neurosensory retina rather than vitreous traction.8 Histopathologically, a retinal hole is the result of a loss of retinal tissue around a sharply limited thinning.8 The chance of a retinal detachment developing from an atrophic retinal hole is between 5 and 10 %.9 There is no consensus on prophylactic treatment of atrophic retinal holes.10,11,12,13 In principle, however, recently developed retinal holes should be treated in symptomatic patients, as they represent a risk factor for retinal detachment.13 The conspicuous symptoms here include, among other things, the occurrence of photopsia and floaters.14 Possible treatment options are laser- or cryocoagulation.13,15,16 In both laser- and cryocoagulation, fresh scars form a few days after the treatment, which gradually pigment. A prerequisite for successful laser- or cryocoagulation is that the retina is still in contact with the retinal pigment epithelium.
Choroidal nevi
A choroidal nevus is a benign intraocular tumour with an approximate worldwide prevalence between 0.4 % and 5 %.17,18 In Australia, Keel et al. found a weighted prevalence of 2.1 % among the non-indigenous population older than 50 years of age. The mean maximum diameter of the nevi in this study was 1.730 mm, the surface area was 2.7668 mm2 and the distance of the nevi from the optic disc was 3.400 mm.19 Raval et al. performed a cross-sectional and follow-up study with 5533 children aged 6 months to 18 years which showed that the prevalence was of 0.47 % in the age group < 6 years, 0.63 % in the age group with 6 years, 1.06 % in the age group with 12 years and 1.79 % in the age group with 18 years.20 Choroidal nevi usually have a diameter of less than 5 mm, have an oval or circular shape, are flat or minimally raised and have a slate-grey appearance.21 In clinical practice, a choroidal nevus is often a chance finding in the course of a retinal examination. Although a choroidal nevus does not change in the long term in most cases, there is a risk of it becoming a malignant choroidal melanoma. In a recent publication, Damato presents a scoring system he has developed to assess and evaluate the referral relevance of melanocytic tumours. Based on this scoring system, tumours can be classified as „ordinary nevus“, „low-risk nevus“, „high-risk nevus“ and „probable melanoma“.22 Shields et al. investigated the transformation of a choroidal nevus into a melanoma on the basis of 2514 consecutive cases. The medial basal diameter of the tumour was 5.0 mm, whereas the thickness was 1.5 mm. In 2 %, 9 % and 13 % of the eyes, there was a transformation of the nevus into a melanoma after 1 year, 5 years and 10 years, respectively. A multivariable analysis predicted this transformation into a melanoma and included factors such as tumour thickness greater than 2 mm (p < 0.001), subretinal fluid (p = 0.002), symptoms (p = 0.002), orange pigment (p < 0.001), a tumour margin within 3 mm of the optic disc (p = 0.001), ultrasonographic depression (p < 0.001), and the absence of a halo (p = 0.009).23 Based on a recent publication by Marous et al., there seems to be no difference within different ethnicities regarding the transformation of a choroidal nevus into choroidal melanoma.24 The course of a choroidal nevus can best be documented by means of a photo of the retina. Ultra-widefield scanning laser ophthalmoscopy (SLO) is particularly suitable for peripheral nevi.25,26,27,28 The Optomap ultra-widefield scanning laser ophthalmoscope is a tool that can help distinguish a choroidal nevus from a choroidal melanoma. The Optomap SLO uses a green laser (532 nm) and a red laser (633 nm) in separate channels to create a composite colour image. According to Kernt et al., a distinction between choroidal nevus and choroidal melanoma can be possible by means of the different channels.29 The possible differentiation of both tumours assumes that choroidal melanomas are visible in all channels of Optomap ultra-widefield scanning laser ophthalmoscope, while choroidal nevi are only visible in the red channel. In a comparative study, Kernt et al. found a sensitivity of 76 % and a specificity of 70 % for a classification of the respective tumours based on the different visibility of the tumours in the red and green channels. A sensitivity of 90 % with a specificity of 82 % was obtained using the assessment of the size of the lesion, the edge touching the optic disc and the presence of subretinal fluid.29 According to a recent study by Papastefanou et al., the sole use of the different visibility of the tumours by means of the red/green channels has a limited applicability to distinguish between choroidal nevi and choroidal melanoma.30
Conclusions
Retinal holes and choroidal nevi are often incidental findings in the course of an examination of the posterior eye segment as part of a comprehensive eye examination. It is thus advisable to perform an examination of the anterior and posterior eye segments when prescribing visual aids. This also applies to contact-lens fitting and prescription, especially in connection with myopia, as this condition is often associated with secondary diseases. Although there is no consensus regarding the prophylactic treatment of retinal holes, the risk of retinal detachment developing from an atrophic retinal hole is between 5 and 7 %, which is why a timely referral to an ophthalmologist should be made. The vast majority of choroidal nevi are benign, but here, too, careful consideration is important. In this context, the British ophthalmologist Timothy L. Jackson recommends in the Moorfields Manual of Ophthalmology a photograph of the lesion and an annual check-up by an ophthalmologist or optometrist for nevi with a low risk of transformation into choroidal melanoma. Nevi with a medium risk of transformation into choroidal melanoma should be checked by an ophthalmologist every 4 to 6 months (at least once a year). Direct referral to an ocular tumour centre should be made in progressive nevi with a medium risk of transforming into choroidal melanoma and in cases of high-risk nevi.31
Conflict of interest
The authors have given seminars and workshops for Optos GmbH in the past and have received a honorarium for these activities.
evaluation-ppp. Referencing: 25 September 2022.
