Sequential Multi Modal Optometric Management of Pediatric Nystagmus
Purpose: The purpose of this article is to present multimodal sequencing as a clinical therapeutic approach for the treatment of pediatric patients with nystagmus based on a personal assessment by the author. While several papers have recommended various therapeutic interventions, a sequencing of these multiple therapeutic managements has never been explicit.
Material and Methods: Extensive literature search (e.g., PubMed, Google Scholar), as well as over 20 years of clinical experience have been used to develop a comprehensive clinical optometric protocol for sequencing of available therapeutic optometric management for pediatric nystagmus.
Results: A comprehensive multi-modal therapeutic protocol for pediatric patients with nystagmus is presented. It includes: refraction, contact lenses, biofeedback, vision therapy, low vision, and referrals for ancillary treatment.
Conclusion: For the first time, a detailed and comprehensive clinical protocol for the management of the pediatric patient with nystagmus has been presented. This should allow for improved patient care, especially with respect to the developmental aspects of nystagmus to provide optimal vision early in life.
Introduction
Nystagmus refers to the involuntary movements of the eyes, typically 2 – 5 Hertz (Hz) with an amplitude of 1 – 5 degrees.1 The annual incidence of pediatric nystagmus varies among different studies. For example, Nash et al. found that in the pediatric population younger than 19 years of age, there was an annual incidence of 0.007 %.2 The Baltimore Pediatric Eye Disease Survey found an overall prevalence of 1.96 % in children 6 months through 71 months of age.2 In 87 % of the cases, the age of onset was by 6 months.3 The most common forms of acquired nystagmus are attributed to with retinal / optic nerve disease (32.4 %) while congenital motor / idiopathic nystagmus represents 31 % of patients with nystagmus.2 It is therefore imperative that a comprehensive and thorough vision evaluation, including the possible etiology and other historical aspects of the nystagmus, be performed, as retinal and neurological disease must be taken into account in the management process.2,4 In some cases, where the nystagmus was secondary to medical conditions such as retinoblastoma or Leber’s hereditary optic neuropathy (LHON) successful reduction of the nystagmus with surgical and or medical intervention such as gene therapy has been reported in the literature.5-7
There are two reasons that necessitate vision intervention for patients with nystagmus. The first is obvious, namely visual function. Both visual acuity and contrast sensitivity can vary considerably in this patient population resulting from the abnormal rhythmic eye movements producing large, abnormal fixation.1 Patients with either congenital retinal or optic nerve disorders (e. g., optic nerve head hypoplasia, foveal hypoplasia, retinopathy of prematurity, etc.) typically have poor visual acuity during infancy and show only minimal change with increasing age.4 In patients with idiopathic nystagmus, visual acuity is mildly reduced during infancy, but there is a failure in emmetropization rates as compared to their peers, hence resulting in relatively poor corrected visual acuity.8,9 The second is the profound psychological effects of the presence of nystagmus, which can have lasting effects into adulthood.10-11 While often times these children are visually asymptomatic, they are often asked, “What is wrong with their eyes,” and teased by their peers, as well as receiving the educators’ negative statements towards the child’s academic potential as they do not understand nystagmus and its impact or lack thereof on academic performance.11 It is therefore imperative to utilize as many treatments available compounding synergistic effects to create the best possible visual outcome for children. The sequencing of treatment options, provides a step by step guide to clinicians to utilize the full armamentarium of possible managements.
Based on existing clinical guidelines, there are three main therapeutic management options to improve vision and reduce the nystagmus, as well as the associated abnormal head posture.4 The three main treatment modalities include: surgical, medical, and optometric, with optometric being the least invasive and with least risk.12,13 It has been postulated that a reduction in nystagmus will yield increased foveation time resulting in improved visual acuity outcomes.14 Steven’s et al. reported that patient with abnormal head posture had better visual acuity as compared with normal head posture.14 The abnormal head posture is an adaptation in order to shift eye position to the null point thereby decreasing the nystagmus while increasing foveation time and improving visual acuity and is a good prognostic sign.14
Surgical Intervention
Surgical intervention has been the mainstay treatment of patient with nystagmus who develop abnormal head poster (AHP) or have concurrent strabismus.15 The surgery for infantile nystagmus is mainly performed to reduce the torticollis by shifting the null point closer to the primary gaze resulting in a decrease of nystagmus.16 While surgery has been demonstrated to be helpful to improve head position, it has not been shown to improve visual acuity consistently with some studies showing no improvement while others showing an average improvement of one line of visual acuity and patients with ocular albinism having no change in visual acuity.15-18
Medication
Several medications have been proposed in the management of symptomatic, acquired nystagmus. Medications such as baclofen and clonazempam can be of used for controlling eye movements, while studies on memantine and gabapentin have shown improved eye movements with decrease of amplitude of nystagmus and improved foveation, as well as improving visual acuity.19-21 However, these medications usually come with a significant list of side effects (e.g., rapid eye movements, difficulty thinking, mood changes) and therefore should be used with caution in the pediatric population.20 The effects are also not long-lasting.
Optometric Management
Optometric management of patients with nystagmus is the least invasive with a low risk for side effects. Traditional optometric management of patients with nystagmus includes glasses, prism, contact lenses, both soft hydrogel and rigid gas permeable lenses. Additionally, adjunctive therapy such as biofeedback has been reported in the literature.22-25
Universally, the first step across the board is optimal spectacle refraction. Clinical studies indicate that these patients have a high prevalence of hyperopia and astigmatism.26,27 The key, however, is an inadequate emmetropization process in this patient population.28 Children with nystagmus have a poor quality of retinal image, and this in turn provides a poor visual signal with respect to feedback required for appropriate axial length growth, and hence the emmetropization process is impeded. In contrast to visually normal children, where some can delay prescribing glasses in anticipation of the normal emmotropization process,29 children with nystagmus, require early intervention with optimal spectacle correction as emmotropization is not expected.
Several eye conditions associated with nystagmus, including aniridia and ocular albinism, have been associated with photosensitivity. The use of photochromatic lenses or prescription sunglasses would be of benefit and should be addressed at the initial visit.
In addition to careful refraction, the use of prism has been advocated for, in the management of nystagmus.22 Yoked prism, where the bases are in the same direction in each eye, can be used to shift the image to the null point, thus dampening the nystagmus23-25. Additionally, yoked prism can be used to improve abnormal head posture.23 While yoked prism has not been found to have a significant effect in normal patients’ head posture, it has not been studied in patients with abnormal head posture.30 Several case reports have noted an improved head posture as well as relief of visual symptoms with yoked prism.31 Base out (BO) prism can also be used to dampen the nystagmus in congenital nystagmus through convergence.23-25 While convergence has been associated with dampening of nystagmus, either through use of overminus lenses or BO prism, it is important to note that lenses and prism not only impacts the nystagmus but also the accommodative system and can likely cause visual discomfort. Overminus has been associated with increasing myopia in the treatment of exotropia, while BO prism has been shown to impact accommodation via convergence accommodation, and furthermore it has been associated with increase of axial length.32-34 Therefore, yoked prism to position the image in the null point is the preferred therapy to BO prism. While surgical procedures have also resulted in improved head posture in patients with nystagmus, yoked prism may be the preferred method. Surgical intervention requires general anesthesia. Xiao et. al’s systematic review revealed that general anesthesia has negative neurocognitive effects in children including effecting academics, cognition and behavior.35 As such it general anesthesia should be used judiciously. Non-invasive techniques for improving head posture should be pursued before surgical procedures.
Contact lenses have long been used as a treatment option of nystagmus.36-38 Contact lenses are more advantageous as compared with spectacle correction, as they improve the optical integrity by minimizing prismatic side effects and peripheral aberrations.36-38 Recent studies indicate that both soft hydrogel and rigid gas permeable contact lenses have no more risk for adverse reaction (e.g., microbial keratitis, corneal inflammatory events) in the pediatric patient population than in the adult population, and contact lenses are routinely recommended for aphakic infants of less than one year of age.39-41 Once a patient is fitted successfully with a spherical contact lens, any residual astigmatism can be corrected by additional spectacles to maximize visual acuity. The spectacle lenses may also contain any yoked prism necessary to reduce the amplitude of nystagmus or to improve abnormal head posture. In patient with aniridia or albinism, artificial iris contact lenses may reduce light sensitivity,42 and improve the quality of the retinal image. Contact lenses also provide tactile biofeedback.
Biofeedback is now recognized as a treatment option for a wide spectrum of medical conditions, including nystagmus. External feedback enables the patient to become aware of physiological functions of which they are normally not conscious. Furthermore, it provides a mechanism of information to improve the physiological function and obtain control where previously there was none. Providing instantaneous feedback through ancillary senses, such as the sense of touch, audition, and vision.43-48 The movement of the lens is felt by the palpebral conjunctiva, via the ophthalmic division of the trigeminal nerve.43,46 This provides tactile feedback to the patient to make the patient cognizant of their abnormal nystagmus eye movement. If the patient can reduce the sensation felt on the palpebral conjunctiva, they are affectively reducing the amount of nystagmus.47
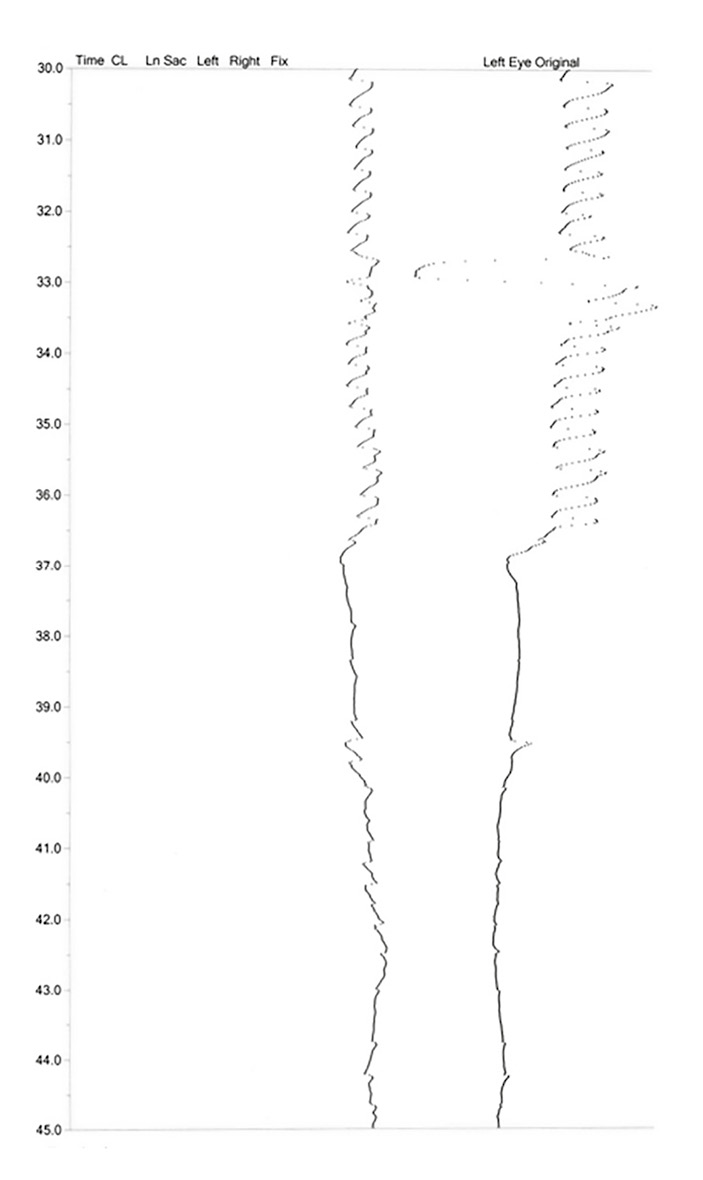
While auditory biofeedback devices are not readily commercially available, they have been shown to be successful in the management of nystagmus by reducing both the amplitude and the frequency of the oscillatory eye movements as well as by improving visual acuity, with lasting results.44,49 A recent pilot study by Daibert-Nido et al. evaluated the use of the audio-visual biofeedback training module of the Macular Integrity Assessment (MAIA) TM microperimeter with promising results in a pediatric population.50,51 Visual biofeedback training can also be successful in children.52 The use of the ReadalyzerTM, (Figure 2) a commercially available eye movement recording system, has been used to provide direct and instantaneous visual biofeedback by having the patient view their eye movements in real time via the device, and thus modify their eye movements to result in a “straighter appearing line” (Figure 3), which translates into reduced nystagmus frequency and amplitude.53-54 Table 1 gives step-by-step instruction with respect to use
this technique.
Table 1: Protocol for use of Readalyzer as a Visual Biofeedback System (adapted from Tannen group 54,55)
- Turn on the Readalyzer and connect the googles
- Adjust the googles to the patient’s pupillary distance
- Set the Readalyzer to record eye movements
- Patient is seated 57 cm away from the screen
- A clear overlay with a central “X” and a “L” 5 cm to the left and an “R” 5 cm to the right of the “X” is attached to the top of the computer
- Patient will be able to see their eye tracing while they are looking at the target
- Have the patient fixate on the X for 3 – 5 seconds
- Have the patient look to the “L” and fixate for 3 – 5 seconds
- Have the patient look to the “R” and fixation for 3 – 5 seconds
- Steps 7 – 9 is the calibration of the system with “L” and “R” being 5 degrees to either side of the “X” giving an estimate of the amplitude of nystagmus
- Patient is then asked look at the center “X” while being aware of the eye tracing off centered
- Patient is asked to attempt to make the lines less jagged
- Patient is instructed to “look softly” with peripheral awareness
Vision therapy administered to patients with nystagmus has been shown to improve both binocular function, as well as oculomotor control.55 The literature is equivocal whether or not oculomotor dysfunction has an impact on reading speed in patients with idiopathic nystagmus.56,57 However, a large subset of these patients have comorbid conditions including strabismus that may benefit from vision therapy.58,59 The literature for vision therapy for nystagmus is limited mostly to case studies.45 Vision therapy is focuses on improving binocular fixation through oculomotor work including fixation, pursuit and saccades. This is accomplished through activities such as Marsden ball and pegboard rotators, visual tracing, line counting, as well as fusional activities.22,45 New computer training systems for improving visual acuity in amblyopia, such as NeuroVisionTM, via, may also help to improve visual acuity in patients with nystagmus.60 However, large scales studies need to be conducted.

Material and Methods
The proposed protocol is based on several sources of information. Extensive searches of the literature via Pubmed, Google Scholar, APA PsychNet, and Semantic Scholar over the past year, as well as related textbooks. While there were over 20,000 citations for nystagmus, there is approximately 4000 articles that on nystagmus in the pediatric population. Approximately 10 articles evaluated biofeedback of various mechanism in nystagmus and approximately 40 related to contact lens use in nystagmus. We included over 50 of those articles in this paper. Where clinical trials were available they were referenced over case reports. However, where large scale clinical trials were not available, such as in the discussion on vision therapy, case reports were included.
Results
A summary of the five-step sequencing of the therapeutic process is presented in Figure 1.
- Refraction is essential to determine the patient’s best corrected visual acuity at distance and at near. This should include an evaluation for sunglasses and tints in patients with photosensitivity related to co-morbid conditions such as ocular albinism or aniridia.
- Assessment of yoked prism to shift the image to the patient’s null point. This can be trialed with Fresnel prism adhesive film on regular glasses to evaluate the patients signs, head position and symptoms over time. The amount of prism prescribed is done based on trial and error with the minimal amount that results in improved head posture and dampening of nystagmus. If the head turn is the right, the null point is in left gaze. Therefore, base right yoked prism would be prescribed (base out in front of the right eye and base in in front of the left eye). Similarly, if the head posture tilted down, with the chin towards the chest, base down prism can be used to decrease the nystagmus and improve head posture. The amount of prism is assessed empirically with trial and error with the aim to prescribed the least amount of prism that is tolerated for effect. Once the magnitude of prism diopters is confirmed, prism lenses can be prescribed noting that the patient may need different prismatic corrections at distance and at near.
- Contact lenses is the next step in this multi-step process with residual correction incorporated into the spectacles if needed for maximum correction. In addition, the aforementioned prism and sunglasses can be incorporated into spectacles that can be worn over the contact lenses. Consideration for tinted or iris painted contact lenses should be considered in those patients with photosensitivity.
- Biofeedback is the next step in this sequence. Which can be tactile, auditory and / or visual. Tactile biofeedback can be accomplished in conjunction with the fitting of either hydrogel and rigid gas permeable contact lenses which provides continuous feedback. Additionally, tactile feedback can also be utilized through digital assessment where patients gently place their finger to their upper eyelid to monitor their eye movements while trying to obtain upper level control.45,46 Auditory biofeedback devices provide the patients with an external sound that represents their eye movements. The steadier the tone, the less nystagmus the patient is exhibiting. Auditory biofeedback systems are no longer readily commercially available. More recently, biofeedback has been used with the MAIA microperimeter. The microperimeter is a device that assesses light sensitivity but uses an eye tracker that can be correlated with a retinal image. The device has an auditory signal related to eye position. Visual biofeedback occurs when they patient can see a representation of their eye movements. Flashed induced foveal afterimage can be used to give patient perception of their eye movements with the goal to reduce the oscillation of the eye movements.22,45 The ReadAlyzerTM is a commercially available eye movement recording device. It is used to evaluate and analyze eye movements during reading. However, the device is also capable of recording eyes. The patient can view their eye movements in real time and with visualization and relaxation aim to reduce the waviness of the recording. A detail of the set up for the readAlyzer is included in Table 1.
- Vision therapy is the next step in the sequence. The aim of the treatment is to improve visual skills. In initial phase of therapy, the emphasis on fixational stability. This is important as poor fixation and saccadic intrusions can complicate and cause the nystagmus to appear worse than it is. Traditional oculomotor activities including mardsen ball, visual tracing, line counting and mazes can be used. Additionally, eye movements therapy is extended to saccades and pursuits using hart chart, Sanet Visual Integrator and similar activities. The use of low contrast target discrimination and line counting with fine separation and decreasing contrast over time would be the next level for oculomotor therapy. It is important to note that comorbid sensory-motor deficits can exist such suppression. Vision therapy can improve suppression through anti-suppression activities such as red green glasses and acetate, vectograms, tranaglyph and anaglyph and improve the nystagmus.
- The final step is making appropriate referrals, for example for low vision services which may include assisted technologies (e.g., telescopes, OrCam) improve visual function and enhance activities of daily living. Low vision management, although is not invasive is listed last. As the child’s visual acuity improves with the other treatment modalities the need for low vision aids will change. As these devices are typically, out of pocket cost, when possible they should be reserved when maximum visual acuity is reach with the various treatment modalities. If the abnormal head posture cannot be corrected with prism, if the patient is symptomatic or if the nystagmus is acquired a referral for surgical or pharmacological intervention may be indicated where specialists should made their decision on a careful assessment of the risk to benefit ratio.
The sequence proposed is a general guideline beginning with options that pose the least risk to that of increased risk. Spectacle correction should always be explored first with contact lenses as soon as the child and / or parent are ready with the assurance that it poses no additional risk to the child. The use of contact lenses effectively begins the first step in biofeedback and readies the child for additional feedback managements as well as vision therapy The goal is to obtain the maximum visual acuity as early in life as possible, and in the shortest possible time frame. Multiple treatment modalities can potentially have a synergistic effect on vision.

Discussion
A multi-modal approach has been widely used for a variety of conditions. It is intended for optimization of treatment where the condition can be targeted for improvement where one modality may potentiate the effect of another. The proposed sequence while yet unproven provides a systematic way of treating nystagmus. Visual task such as vision therapy cannot be achieved without optimal correction. Optimal correction begins with spectacles and evaluation of prism correction. Introduction of contact lenses should be done as early as possible but this process often takes time and the child should be in correction during the process. The process of biofeedback begins with the contact lens. Increased feedback awareness, even with simple tactile feedback with a finger can give a child who is otherwise asymptomatic indication of their eye movements. Finally, vision therapy can be added to management to improve additional oculomotor and sensory defects.
Conclusion
Currently, it is not possible to formulate evidence based recommendations for standard based sequencing of management of childhood nystagmus. Prospective studies to compare individual managements and management strategies should be evaluated to determine the cumulative benefits and speed of improvement. The utilization of yoked prism in comparison to surgical intervention for abnormal head posture should be compared. Additionally, a comparison of visual biofeedback and classical vision therapy effect on foveation time and foveation time’s impact on visual acuity in a double blind randomized clinical trial should be tested. The challenge is to design studies with enough patient sampling to see the effects of each individual treatment and then those treatments in combination. Nevertheless, until the research can provide guidance, it is the opinion of this article that providers educate both themselves and their patients to the range of rehabilitative modalities available to them to improve their visual outcomes and potential to ameliorate psychological aspects related to nystagmus.
Conflict of interest
The author has no conflict of interest with regard to the methods and devices mentioned in the article.
Article for download
Download here: Sequential Multi Modal Optometric Management of Pediatric Nystagmus
Dizhoor, A. M., Boye, S. E. (2021). Safety and improved efficacy signals following gene therapy in childhood blindness caused by GUCY2D mutations. iScience, 24, 102409.
